Summary
Ischemic postconditiong (postcon) has been reported to reduce infarct size in ST-segment myocardial infarction (STEMI). However, recently a few other studies did not show any effect of postcon or suggested that it may even be harmful. The aim of the study was to assess whether postcon could reduce infarct size (IS) and the microvascular obstruction (MVO) zone in early presenters with STEMI. Patients with STEMI treated with primary coronary intervention (PCI) < 4 h from symptoms onset were randomly assigned to the postcon group or standard PCI group. Postcon was performed immediately after obtaining reperfusion with 4 balloon occlusions, each lasting 60 s, followed by 60 s of reperfusion. Cardiac magnetic resonance was performed in all subjects within 48 to 96 h after admission. Postcon was associated with significantly smaller IS and higher ejection fraction. The extent of MVO was significantly lower in the postcon group in comparison to the control group. In early presenters with STEMI postcon could significantly reduce infarct size and limit reperfusion injury.
Introduction
Effective reperfusion therapy has been shown to reduce the myocardial necrosis and significantly improve the prognosis in patients with acute ST-segment elevation myocardial infarction (STEMI) [1]. However, restoration of blood flow in the infarct-related artery (IRA) after prolonged occlusion may also have deleterious effects, including extension of the myocardial necrosis zone, the no-reflow phenomenon and reperfusion arrhythmias, making reperfusion therapy a “double-edged sword” [2, 3]. Ischemia-reperfusion injury is a complex phenomenon mediated by several factors, including oxidative stress, intracellular calcium accumulation, rapid restoration of pH, and inflammation, and involves, at least partly, opening of the so-called mitochondrial permeability transition pore (mPTP) [2–8].
Ischemic postconditioning (postcon), defined as repetitive interruptions of the coronary blood flow applied after a period of ischemia, has been thought to inhibit reperfusion injury and has been shown to reduce infarct size in patients with STEMI [9–18]. However, some newer studies did not show any effect of ischemic postcon and even suggested that it may be harmful [19–24].
Microvascular obstruction (MVO) after primary percutaneous coronary intervention (pPCI) for STEMI is a sign of the no-reflow phenomenon in coronary vessels smaller than 200 µm despite adequate epicardial reperfusion. MVO is generally thought to be related primarily to reperfusion injury [25]. Cardiovascular magnetic resonance (CMR) provides unique characterization of postinfarctional myocardial injury. The presence of MVO was shown to be associated with larger infarct size, negative left ventricular remodeling and adverse cardiovascular outcomes [26].
Larger infarcts on CMR are consistently associated with larger ventricular volumes, lower ejection fraction and greater MVO, which occurs in 40–60% of patients treated by primary percutaneous coronary intervention. The influence of postcon on the presence and extent of MVO and its relations with the myocardial necrosis have been studied in only one small clinical trial [27].
Aim
The present study was designed to test the hypothesis that postconditioning reduces the extent of myocardial necrosis and the MVO zone observed in CMR in high-risk STEMI patients.
Material and methods
Study design and protocol
This was a prospective, single-centre, randomized, controlled, open-label study with blinded evaluation. The study was performed according to the provisions of the Declaration of Helsinki. All the participants gave their written informed consent for the investigation. The protocol of the study was approved by the Local Ethics Committee (No. 547/11).
From October 2010 to January 2015 all patients admitted to our department who were eligible for a primary PCI due to STEMI were considered for enrolment in our study, provided they fulfilled the following inclusion criteria: age > 18 and < 80 years old, chest pain > 30 min and < 6 h (preferably < 4 h, < 6 h only if persistent chest pain and ST-segment elevations), ST segment elevation > 0.1 mV (> 0.2 mV in V1–V3) in two contiguous ECG leads, and a thrombolysis in myocardial infarction (TIMI) grade 0 flow in the infarct-related artery. The exclusion criteria were as follows: previous myocardial infarction, previous coronary artery bypass surgery, cardiogenic shock, cardiac arrest, the presence of collateral flow to the infarcted area as evidenced by a Rentrop score ≥ 1, known renal impairment (serum creatinine > 150 mmol/l), persistent atrial fibrillation, ongoing malignant process, a history of gastrointestinal bleeding or stroke, any contraindication for GP IIb/IIIa inhibitors, contraindication to cardiac magnetic resonance examination including claustrophobia, implanted electrotherapy devices and any condition that was considered to interfere with the possibility for the patient to complete the study protocol.
All patients received aspirin (300 mg orally), clopidogrel (600 mg orally), and heparin (intravenously at a dose of 70 UI/kg) before PCI. Coronary angiography was performed by the percutaneous technique using the transradial approach in most cases and the infarct-related artery was identified. Following angiographic data acquisition, patients were randomized, using a 1 : 1 sequence placed in numbered sealed envelopes, into the control or postcon group. After guidewire placement in the distal part of the vessel, manual thrombectomy was performed in all patients. In the control group a drug-eluting stent was subsequently implanted with direct technique whenever possible. Postconditioning was performed by reinflating the balloon at the same location to a pressure 4–6 atm for 60 s starting 1 min after initial reperfusion with thrombectomy. This cycle was then repeated four times. The balloon was located proximally to the site of the index lesion (to avoid embolization with the elements of ruptured plaque). The selection of the inflation-deflation sequence was based on previous experimental and human studies [8, 9]. Patients in the control group received no additional intervention during 4 min of reperfusion. The procedure was finished with drug-eluting stent implantation. IIb/IIIa inhibitors (abciximab or eptifibatide) were administered in all patients. All patients were treated with aspirin 75 mg daily lifelong and clopidogrel 75 mg daily for 12 months.
Angiographic analysis
Angiographic assessment was performed independently by two experienced angiographers who were blinded to each other and to the clinical data. It included TIMI flow grade before and after the procedure, myocardial blush grade (MBG) and Rentrop’s score of collateral flow.
CMR image acquisition
CMR was performed on all the subjects on a 1.5 T scanner (Siemens Avanto, Erlangen, Germany) 48–96 h after admission. Cine imaging with steady-state free precession (SSFP) and late gadolinium enhancement (LGE) imaging were performed in long-axis views and contiguous short-axis slices covering the entire left ventricle (LV). LGE images were acquired 10–15 min after contrast administration using a segmented inversion-recovery gradient-echo sequence.
CMR image analysis
Analysis was performed offline blinded to patient details using QMass 7.1 (Medis, Leiden, Netherlands) by two experienced observers. LV volumes and function were calculated as previously described. The infarct zone was defined semi-automatically on LGE imaging using the full-width half-maximum (FWHM) technique. LGE areas (infarct size – IS) were defined as areas with a signal intensity of mean > 5 SD of visually normal, contralateral remote myocardium, as described previously. MVO was defined as areas of hypoenhancement within the infarct zone and was included in the assessed IS. Total MVO volumes and IS volumes were calculated from manual planimetry of short-axis images by the summation of discs and were expressed as IS and MVO volumes and mass, as well as LV myocardial/IS or MVO ratios (Figure 1).
Figure 1
Cardiac magnetic resonance late-gadolinium enhancement (LGE) images in a patient with inferior wall STEMI. A, B – Infarct size (IS) is an area greater than 50% of maximal signal intensity. Microvascular obstruction (MVO) is an area of hypoenhancement surrounded by LGE (white arrows). C – Infarct size and MVO were determined by planimetry and the summation of discs method
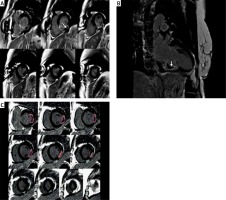
IS and MVO and LV volumes were also indexed by body-surface area.
Study endpoints
Study end-points were IS expressed as volume and mass, and MVO zone expressed as volume and mass in CMR performed 48–96 h after intervention.
Statistical analysis
Calculation of sample size was performed according to the previous studies [27]. Considering an expected reduction of 30% in the MVO extent with statistical power of 80% and a probability of type I error of 0.05 with 2-sided test, we calculated a total sample size of 50 patients. To compensate for patient dropout, a total of 74 patients were recruited.
Comparisons between groups were performed using unpaired Student’s t-test or the Mann-Whitney U-test for continuous variables and χ2 or Fisher’s exact test for categorical variables. A value of p < 0.05 was considered statistically significant.
Results
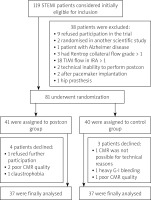
From a total of 486 screened STEMI patients, 119 patients met the initial criteria and were found initially eligible for the study, but 38 were subsequently excluded as shown in Figure 2. The remaining 81 patients underwent randomization to postcon and control groups, but 7 declined participation, which left 74 patients (37 in the postcon group and 37 in the control group). There were no significant differences between the groups with regard to baseline demographic characteristics (Table I). In postcon patients MBG 3 after PCI was observed significantly more often in comparison to the control group. Angiographic and procedural characteristics are shown in Table II.
Table I
Clinical characteristics of study groups
Table II
Angiographic characteristics of study group
Postcon was associated with significantly smaller IS (16.6 ±9.7 vs. 31.2 ±22.9 g; p = 0.0004) and higher left ventricular ejection fraction (59.8 ±9.2% vs. 52.3 ±10.2%, p < 0.05) (Table II). IS/LV myocardial mass was also significantly lower in the postcon group in comparison to the control group (Figure 3).
Figure 3
Differences between infarct size (IS) mass, infarct size to left ventricular (LV) myocardial mass index and micro-vessel obstruction (MVO) mass, MVO to LV myocardial mass index
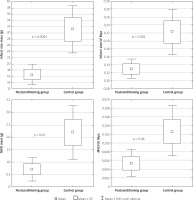
The extent of MVO was significantly lower in the postcon group in comparison to the control group (0.71 ±1.4 g vs. 2.2 ±3.2 g; p = 0.03) (Table II). However, differences in the MVO/LV myocardial mass ratio did not reach statistical significance (p = 0.06) (Figure 3).
We observed lower creatinine kinase, and troponin I peak serum levels (2297 ±1391 vs. 3268 ±1163 U/l; p < 0.001 and 47.4 ±57.1 vs. 136 ±122 ng/dl; p < 0.01) in the postcon group compared to the control group (Tables III and IV).
Table III
Parameters of cardiac magnetic resonance in study groups
Table IV
Laboratory markers of myocardial necrosis in postcon group and in control group
Discussion
This prospective randomized study evaluating the effect of postconditioning in STEMI patients treated with pPCI showed that postcon significantly decreased IS and MVO assessed by CMR.
The concept of postconditioning was described for the first time by Zhao et al. [9] in a canine model. The authors documented the effectiveness of intermittent episodes of ischemia and reperfusion applied after prolonged closure of the infarct-related artery. The left anterior descending artery in dogs was ligated for 45 min and then there were performed intermittent periods of reperfusion and reocclusion (3 × 30 s). Forty-four percent reduction in infarct size as compared with the control group was observed [9].
A few postulated mechanisms of cytoprotective effects of postcon exist. It has been shown that modification of reperfusion affects important mediators of lethal reperfusion injury, reducing oxidative stress, rapid washout of adenosine, osmotic shock and intracellular calcium ion overload [8, 28–30]. Postcon also improves endothelial function, reduces cardiomyocyte apoptosis, reduces infiltration of neutrophils and, in particular, delays the restoration of neutral pH [28–31]. Postcon causes an increase in the extracellular space of endogenous ligands, such as adenosine, opioids, bradykinin and PAR-2 agonists (protease activated receptor-2), which, acting on specific membrane receptors, triggers multiple signaling pathways [29–33]. Postcon is a strong impulse, which activates the reperfusion injury salvage kinase (RISK) pathway [32, 33]. This leads consequently to the closure of the key mPTP channels in the mitochondrial membrane [29–33].
Soon after the Zhao et al. publication, the first clinical application of postcon in patients treated with primary percutaneous coronary intervention was made [9]. Studies conducted initially in small groups of patients were promising. Several different algorithms of intermittent reperfusion – 3 × 30s, 3 × 60s, 4 × 30s, 2 × 90s – were applied [11–18]. In small studies of less than 30 patients postcon during primary PCI seemed to protect the human heart during STEMI, showing a 36% infarct size reduction as determined by cardiac biomarkers and improved coronary flow reserve and ST-segment resolution [10, 11]. Similarly, Yang et al. reported a 27% reduction in IS in 41 STEMI patients treated with primary PCI plus postcon as assessed by scintigraphy [13]. The significant influence of postcon on periprocedural ventricular arrhythmias has also been reported [34]. In a randomized study, Lønborg et al., using magnetic resonance, reported that postcon resulted in a 19% relative reduction of the infarct size in relation to the area at risk [18]. Recent studies, however, did not confirm the cytoprotective effect of postcon and weakened the enthusiasm for this method. In a few studies it was found that the infarct size did not differ significantly between the two groups, while in two others postcon proved to be even harmful [19–34]. In a trial which included 700 patients, Hahn et al. [19] found that postcon did not improve myocardial reperfusion in patients with STEMI undergoing PCI with the current standard practice (small size balloon predilatation or thrombus aspiration and stenting). In the biggest randomized trial to date, DANAMI 3-iPOST, Engstrøm et al. found that ischemic postcon in 1234 randomized STEMI patients hospitalized up to 12 h from symptoms onset failed to reduce the composite outcome of death from any cause and hospitalization for heart failure [20]. Kim et al. [21] in a CMR subanalysis of POST trial showed no benefit of ischemic postcon as an adjunctive treatment of primary PCI. Moreover, Freixa et al. [22] observed in a group of 79 patients that postcon was associated with lower myocardial salvage and lower myocardial salvage index assessed in CMR 1 week after STEMI. They found no significant differences in infarct size and left ventricular ejection fraction between the groups at 1 week and 6 months after MI. The authors suggested that postcon during primary PCI does not reduce infarct size or improve myocardial function recovery at both short- and long-term follow-up and might have a potential harmful effect [22]. In the POST-AMI trial, Tarantini et al. randomized 78 patients with STEMI to the postcon or PPCI plus abciximab group [23]. The authors observed that postcon did not have the expected cardioprotective effect and on the contrary it might harm STEMI patients treated with primary PCI plus abciximab. These contradictory findings may be attributable to differences in PCI procedures [24, 35]. Studies in which only a direct stenting technique was allowed or predominantly performed demonstrated the beneficial effect of ischemic postconditioning, whereas studies in which primary PCI was performed according to the current standard practice such as predilatation with small-sized balloons failed to demonstrate the beneficial effect of postcon [35]. In our study all the patients underwent manual thrombectomy as well as intravenous administration of GP IIb/IIIa inhibitors. Although this type of treatment is no longer used at present, when recruiting patients for the study it seemed to be an optimal reperfusion strategy in combination with primary coronary angioplasty.
One should also note that primary coronary angioplasty of the infarct-related artery can itself become a kind of postconditioning. Pre-dilation cycles, dilatation of the balloon during stent implantation and finally postdilatation of the stent with the non-compliant balloon may act cardioprotectively; hence there is no difference between the patients treated with postcon and the standard primary PCI group [35].
In the present study a significant reduction in IS (by 47%) was observed; moreover MVO zone, related primarily to reperfusion injury, was also significantly smaller in comparison to the control group. These observations are in line with earlier clinical experiences looking at the impact of postcon on MVO. Mewton et al. [27] found in 50 STEMI patients that postcon was associated with smaller early and late MVO size. This significant reduction was persistent after adjustment for thrombus aspiration, which had no significant effect on either infarct size or on early or late MVO. Attenuation of MVO was associated with infarct size reduction. The authors concluded that mechanical postconditioning significantly reduces MVO in patients with acute STEMI treated with primary angioplasty. In a recent study Traverse et al. observed that postcon had no early beneficial effect on infarct size or left ventricular function compared with routine PCI. However, postconditioning was associated with improved left ventricular remodeling at 1-year follow-up, especially in subjects with the presence of MVO [36].
The protocol of the study may also have a considerable impact on the effectiveness of postcon. Thus, when planning the study we made sure to implement an optimal reperfusion therapy. All our patients received IIb/IIIa inhibitors and had the infarct-related artery opened using aspiration thrombectomy. Direct stenting was performed whenever possible to avoid microembolisation, which could grossly deteriorate the beneficial effects of postconditioning.
Undoubtedly, there are some limitations of our study. Despite randomization, as one might expect in a relatively small study, there are some imbalances in the baseline characteristics between study groups, albeit not statistical significant (in myocardial mass index and volume). Although MVO extent was significantly lower in comparison to the control group, MVO/LV myocardial mass was not significantly lower. The results should be interpreted with caution.
Conclusions
Postcon can significantly reduce the infarct size and microvascular obstruction zone in patients treated with primary PCI, although this applies only to early presenters with an extensive area at risk. However, the results should be interpreted with caution and further research is necessary to determine the optimal protocol of the treatment and identify a target group which can best benefit from postconditioning.