Introduction
In patients undergoing curative resection for lung cancer, besides the significant risk of local recurrence, the risk of developing new lung cancer is also higher compared to the general population [1–5]. Regular and close follow-up after curative resection is therefore of great importance. When such new lesions are detected, a good prognosis can be achieved through a second surgical resection in early-stage patients showing no distant metastases and having sufficient cardiopulmonary reserve [5–7].
Aim
In this study, we examined the factors affecting the second surgical procedures performed due to lung malignancy, mortality, and survival rates in patients who underwent radical anatomical lung resection for non-small cell lung cancer (NSCLC), and tried to find some specific clues about the type and extent of the second surgery and patient selection.
Material and methods
We retrospectively analyzed data from a single institution, to identify patients who underwent surgical resection for lung cancer between January 1, 2010, and March 31, 2020. Inclusion criteria were as follows: 1) two separate curative-intent pulmonary resections for recurrent or second primary lung cancer; 2) lesion pathologically confirmed as primary NSCLC. Exclusion criteria were as follows: 1) carcinoid tumors; 2) those who underwent surgery due to pulmonary metastasis of an extrapulmonary malignancy; 3) those with extrathoracic metastasis; 4) those who underwent wedge resection in the first operation.
Operative mortality was defined as deaths before discharge from the hospital or within the first 30 days after the operation. Lesions that were not detected during the first operation and developed in the same or opposite lung within 24 months showing the same histology were defined as locally recurrent tumors. Concurrent tumors, regardless of histology, in the opposite lung without mediastinal nodal involvement, as well as those of different histology (synchronous) occurring within the first 24 months, or those of the same/different histology (metachronous) occurring after 24 months, were defined as the second primary tumor.
In the first postoperative period, patients were followed up by a thoracic surgeon 2 to 3 weeks after resection. Patients underwent radiological follow-up for at least 5 years; every 3 months for the first 2 years, every 6 months for the next 2 years, and then annually. 18 F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) was performed on those with suspicious lesions. Fiberoptic bronchoscopy and/or trans-thoracic fine-needle aspiration biopsy was performed when necessary. A second resection was performed in patients who showed no extrathoracic spread and had sufficient cardiopulmonary reserve if the lesion was only localized to the lung.
All medical records of the patients, operation notes of both procedures, pathology results, and postoperative follow-up records were examined. Survival was calculated by revising the date of death after the second surgery and by updating the records for the survivors on May 1, 2020, from the National Population Registration System, and all deaths were taken into account.
To analyze the condition in young and elderly patients, the patients included in the study were separated into two groups: those under 60 years of age and those aged 60 and over. Forced expiratory volume in 1 second (FEV1%) was classified as below 70%, and 70% and above. In the analysis of patients with comorbidities, chronic lung disease, heart disease, diabetes mellitus, and essential hypertension were taken into consideration. The preferred method of surgery was classified as video-assisted thoracic surgery (VATS) and thoracotomy.
The study was approved by the institutional review board (IRB date/no: 22.07.2020/49109414-604.02-7012).
Statistical analysis
The estimated survival rates were calculated using the Kaplan-Meier method. All deaths excluding operative mortality were taken into account. Variables affecting survival were compared using the log-rank test and Cox regression analysis. Among the groups, univariate analysis was performed using Fisher’s exact test or the Pearson χ2 test, and multivariate analysis was performed using binary logistic regression (forward stepwise). P < 0.05 was deemed significant. Variables with a p ≤ 0.25 in the univariate analysis were included in the multivariate analysis.
Results
Seventy-seven patients were included in the study and the mean age was 63 ±7.4 (46–83) years. In the first operation, lobectomy or bilobectomy was performed on most of the patients, while three patients underwent segmentectomy and one patient underwent pneumonectomy (Table I).
Table I
Patients’ data of the first operation
The mean time between the two operations was 36.7 (1–201) months. Squamous cell carcinoma was the most common among the histopathological types detected in both the first and the second operations (54.5% and 48.1%). VATS was performed in 12 (15.6%) patients for the first operation and 18 (23.4%) patients for the second operation. In the second operation of the patients who underwent VATS in the first operation, VATS was performed in 5 (41.7%) and thoracotomy in 7 (58.3%). In the second operation of the patients who underwent thoracotomy in the first operation, VATS was performed in 13 (20%) and thoracotomy in 52 (80%). Eight lobectomies, 2 segmentectomies, and 8 wedge resections were elected for VATS in the second surgery. Twenty-one completion pneumonectomies, 18 lobectomies, 2 segmentectomies, and 18 wedge resections were performed in patients who underwent thoracotomy in the second surgery. The patient characteristics of the second operations are shown in Table II. After the second operation, operative mortality occurred in 8 (10.4%) patients. Mortality was more common in patients who were old, had comorbidities, had a low FEV1 (%) value, underwent thoracotomy, underwent lobectomy or more extensive resection, and those on whom second surgery was performed in less than 36 months (Table III). However, this difference was statistically significantly higher in patients who underwent the only thoracotomy in both operations than in those who had VATS in at least one operation (p = 0.004) (Table IV).
Table II
Patient characteristics of the second operation
Table III
Characteristics of patients with postoperative mortality
Table IV
Analysis of variables affecting mortality based on the second operations
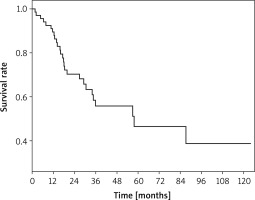
During a mean follow-up of 33.4 months (1.5–123 months), 27 patients died, excluding operative mortality, while 42 patients were still alive. The 5-year overall survival rate was 46.5%, and the mean and median survival rates were 68.5 months and 57.8 months, respectively (Figure 1).
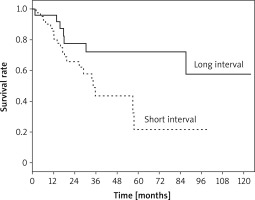
Five-year survival in patients operated on for recurrent and second primary tumor was 32.8% and 51.1%, respectively (p = 0.81). When we examined the subgroups of the second primary tumor, we found that 5-year survival was 65.1% in metachronous tumors, while 4-year survival was 38% in synchronous tumors, and no patients in this group had lived for 5 years (p = 0.078). The 5-year survival rate was 21.8% for patients who underwent two operations within less than 36 months, while it was 72.2% for those who had the second operation after more than 36 months (p = 0.028) (Figure 2). Additionally, in univariate analysis, the survival rate was found to be significantly lower in elderly patients (p = 0.004). In the Cox regression analysis, only age group was identified as a statistically significant variable for survival (p = 0.019; odd ratio (OR) = 3.1; confidence interval (CI) = 1.2–8.0) (Table V).
Table V
Variables affecting survival
Discussion
In parallel with the developments in techniques for lung cancer diagnosis and treatment, the number of patients receiving early diagnosis and treatment is gradually increasing. The number of patients living after curative treatment and their lifespan are also increasing [8]. It is known that these patients have a significant risk of local recurrence and an increased risk of developing new lung cancer [1–5]. Through advanced examination techniques and postoperative close follow-up, potentially treatable, new, and recurrent cancers can be detected at an early stage more frequently [2]. Throughout the study period of more than 10 years, second lung cancer surgery was performed in 4% of 1925 patients who had previously undergone an operation for lung cancer in our clinic. In different series, this rate is reported between 3.1% and 4.1% [3, 5, 6, 9].
Due to the patient’s age, loss of respiratory function caused by the first surgery, side effects related to treatments such as chemotherapy/radiotherapy, if received, and the presence of more additional diseases, the mortality rates of surgical procedures to be performed increase compared to those who have surgery for the first time. In the literature, the postoperative mortality rate is reported to be in the range 0–29%, depending on the diagnosis and extent of resection [1, 3, 6, 10–13]. In our study, this rate was calculated as 10.4%. Similar to the literature, the postoperative mortality rate was higher in our patients who were old, had comorbidities, underwent thoracotomy, had a longer interval between operations, and underwent lobectomy or more extensive resection [14–16]. When we consider the extent of the resection in the second operation of eight patients with postoperative mortality, we see that sublobar resection was performed in only one patient. Lobectomy or pneumonectomy was performed in the other seven patients. However, only those who underwent thoracotomy in both operations had significantly higher postoperative mortality. This can be attributed to the lower postoperative morbidity and mortality in patients undergoing resection through VATS, as well as the fact that VATS is preferred by thoracic surgeons for less risky procedures. Similar to our study, studies comparing VATS with thoracotomy have reported in recent years that postoperative mortality is lower in VATS cases [14, 17].
In many studies in the literature, a second resection has been recommended for selected patients with NSCLC detected after curative lung resection [5–7]. In different series, 5-year survival rates following second resections performed due to various indications are reported to be between 30% and 69% [5, 10, 12, 18]. In our series, the overall 5-year survival rate was 46.5% and age was the only significant variable affecting survival. Okubo et al. [7] suggested that node metastasis, age, and disease-free period affect survival, while Lee et al. [5] reported that the only variable affecting survival is stage. Both studies emphasized that surgery can yield long-term survival in patients with adequate cardiopulmonary reserve. Some studies have argued that it does not affect the prognosis whether the histology is the same or different and whether the lesion is ipsilateral or contralateral [5, 6]. It was also observed in our series that, besides the histological type of the two tumors, whether the second tumor was located on the ipsilateral or contralateral side, whether the FEV1 value was below or above 70%, tumor size, or nodal metastasis does not have any impact on 5-year survival.
Although recurrence is primarily considered for newly developing lung cancer in a patient receiving curative treatment due to primary lung cancer, this may be a synchronous or metachronous tumor. In the literature, 5-year survival was reported to be in the range of 15–43% in recurrent tumors [1, 7, 10, 19], while this rate was 19% to 87% in synchronous and metachronous tumors, and there was no significant difference between the groups in these studies [1, 6, 9, 10, 13, 18, 19]. In our study, this rate was 32.8% in recurrent tumors and 51.1% in second primary NSCLC (p = 0.81). In a recently published meta-analysis, 10 articles discussing synchronous and metachronous tumors were reviewed. According to this analysis, excluding a study specific to tumors with adenocarcinoma histology, the median survival for synchronous tumors was reported to be between 32 and 55 months and 5-year survival was between 19% and 40% [13]. In our series, the median survival for synchronous tumors was 33.9 months, and 4-year survival was 38%. In the same meta-analysis, the 5-year survival of metachronous tumors was reported to be in the range of 32% to 66%, and this rate was similarly 65.1% in our series.
While Okubo et al. [7] reported that the disease-free period between two operations is a poor prognostic factor for decreased survival, different studies argued that the interval between the operations has no significant impact on survival [5, 6, 12]. In our study, the 5-year survival rates calculated for the second surgery performed before or after 36 months were 21.8% and 72.2%, respectively. While this difference was significant in univariate analysis (p = 0.028), it did not emerge as a significant prognostic factor in Cox regression analysis (p = 0.33).
Another disputable issue is the extent of the second surgical resection to be performed. Depending on the location and size of the lesion, completion lobectomy or completion pneumonectomy may be required in the second operation; however, sublobar resections may also be preferred in early-stage patients. In our study, limited resection was preferred in eligible patients in the second operation as much as possible. These patients had a better, although not statistically significant, survival rate compared to those with more extensive resections. The 5-year survival rate was 56.7% for sublobar resection, and 36.3% for lobectomy or more extensive resection (p = 0.24). It has also been reported in the literature that more limited resections can be performed with similar survival rates in eligible patients [5, 9, 18].
Our study has some limitations. Firstly, this is a retrospective study. Secondly, this is a single-center study. If we had included more health centers and compared other results, the results would be more objective. Thirdly, the small number of our patients in some subgroups made it difficult for us to analyze some data, although it is not different from the series in the literature.