Introduction
Genitourinary syndrome of menopause (GSM) affects up to 48% of pre-menopause women and up to 90% of menopausal women. Nearly 70% of breast cancer survivors who develop iatrogenic menopause complain of GSM [1]. Menopause is characterized by significant hormonal changes that are responsible for physiological, histological, and anatomical changes that affect the urogenital tract. The epithelium, normally rich in oestrogen receptors, is under hormonal control composed of adequate levels of collagen and polysaccharide that help to maintain its thickness and moisture. In a hypoestrogenic environment, the vaginal epithelium becomes thin, the vagina loses elasticity, and blood flow is reduced [2].
The symptoms can be a burden for the affected woman, leading to psychosocial distress and a negative impact on quality of life [1, 2].
Many menopausal women with complaints of dyspareunia have significant vestibular tenderness due to oestrogen deficiency, which increases the density of sensory nerve fibres in the vulva and the vagina. For this reason, the most recent consensus statement includes GSM as a cause of provoked vestibulodynia (PVD) [3, 4]. Nevertheless, a multifactorial aetiology involving psychosomatic factors, neural sensitisation, and local vaginal changes is the most likely cause of PVD [5].
According to Friedrich [6], PVD is characterized by (1) severe pain upon vestibular touch or attempted vaginal entry; (2) acute pain during cotton swab palpation of the vestibular area; (3) vestibular erythema. The same as GSM, PVD is often associated with comorbid physical and psychological conditions, including depressive and anxiety disorders, and rarely to more serious complications [7].
Few therapies have been shown to be effective for PVD. Current therapeutic approaches include topical treatment and hormones [8, 9]. Non-hormonal lubricants and moisturizers can be safely used also for GSM symptoms, but their real benefit is controversial. Topical hormonal treatment is first-line therapy in GSM, but it has some side effects.
In recent years, laser technology has been introdu-ced and rapidly applied in various fields of medici- ne [10]. Many studies have compared laser CO2 therapy efficacy to local hormonal therapy, proving its cost- effectiveness and safety and showing comparable outcomes in improving GSM and PVD symptoms by increa-sing vaginal health [11–13].
Both first-generation laser (such as fractional microablative CO2 laser) and non-ablative vaginal laser (such as Er:YAG laser) technologies are known for their local ability to stimulate cellular metabolic activity of fibroblasts that synthesize new collagen, hyaluronic acid, and extra cellular matrix, by provoking local inflammation though a pathway that involves heat shock proteins, cytokines, and growth factors [10, 14]. While CO2 fractional laser function with a gas medium is mostly used in dermatologic surgery for skin lesions treatment, Er:YAG laser uses a solid medium and has traditionally been used for dermatologic and dental procedures [15].
Herein we introduce a new non-ablative solid-state laser: Ladylift®. This technology consists of a combination of a non-ablative laser (LASEMAR® 1500) with 3 different handpieces that permit the delivery of energy equally to the target area. The first handpiece (Ladylift® internal handpiece) is used to deliver the energy inside the vagina, with a uniform and continuous emission of 360° on the whole mucosa wall. The second handpiece (Ladylift® Vulvo-perineal Handpiece) is used to deliver the laser emission on the external areas to complete the treatment evenly on all tissues that need rejuvenation. The last, surgical contact handpiece transforms the device into a surgical tool thanks to the focalization of the laser energy in a microscopic area of 1/3rd of a millimetre (300 microns). This handpiece can be used for a wide range of operations: in plastic surgery it can be used for vaginoplasty, labioplasty, laser lipolysis, and fat remodelling, while in the office can be used for vaporization of condylomas, HPV, and warts with the advantage of a sterile and bloodless operatory field.
With this study we want to present a painless treatment protocol, free of side effects, which consists of 4 laser sessions for the duration of a few minutes delivered 2 weeks apart.
Material and methods
This is a prospective study conducted on a sample of private patients by a single operator.
The study was conducted according to the guidelines of the Declaration of Helsinki, and informed consent was obtained from all subjects involved in the study.
We enrolled, between November 2021 and February 2022, 18 post-menopausal women presenting to a private clinic with GSM symptoms and provoked vulvodynia.
Inclusion criteria were: 1) women in postmenopausal age (defined as absence of menstruation for at least 24 consecutive months); 2) women with symptoms of vulvovaginal atrophy; and/or 3) diagnosis of PVD to Friedrich’s criteria [1) severe pain upon vestibular touch or attempted vaginal entry; 2) acute pain during cotton swab palpation of the vestibular area; 3) vestibular erythema].
Exclusion criteria were: 1) complicated vulvovaginal candidiasis (infections in immunosuppressed subjects and chronic fungal infections); 2) recurrent vulvovaginal candidiasis (at least 4 culturally confirmed episodes in 12 months); 3) sexually transmitted infections within the past 6 months; 4) systemic antibiotic or antifungal treatment, whether ongoing or in the 4 weeks prior to entering the study; 5) systemic hormone replacement therapy or use of topical hormone products whether ongoing or in the 4 weeks prior to entering the study; 6) use of topical lubricants or soothing agents whether ongoing or in the 4 weeks prior to entering the study; 7) autoimmune diseases, thyroid diseases, or history of atopy; 8) diabetes mellitus; 9) chronic infections (human immunodeficiency virus – HIV, hepatitis C virus – HCV, hepatitis B virus – HBV). The participants in the study were all between 50 and 58 years old.
Postmenopausal women were treated extravaginally and internally with Ladylift® non-ablative laser technology. The study protocol consisted of 4 treatment sessions, each lasting a few minutes (on average 4 minutes) and repeated 2 weeks apart. Primary outcomes were evaluation of pain related to GSM and PVD symptoms and of vaginal health at base and at follow-up.
The most common tools used worldwide to evaluate pain intensity are the 10-cm visual analogue scale (VAS) and the numeric rating scale (NRS). The numeric rating scale is a segmented numeric version of the VAS, which is easier to manage, in which the patient vocally selects a number from 0 to 10 (0 “no pain”, 10 “worst pain imaginable”) that best reflects the severity of the discomfort perceived [16]. According to the WHO’s pain relief ladder [17], a score of 0 is considered no pain, 0–3 mild pain, 4–6 moderate pain, and 7–10 severe pain.
Before beginning the first session, we assessed the patient’s pain perception related to GSM and PVD symptoms by using the NRS pain scale (NRS-0).
Pain perception was again rated one month after the beginning of treatment (NRS-1) and 2 months after the end of the 4th session (3.5 months after the beginning of therapy) (NRS-2) (Fig. 1).
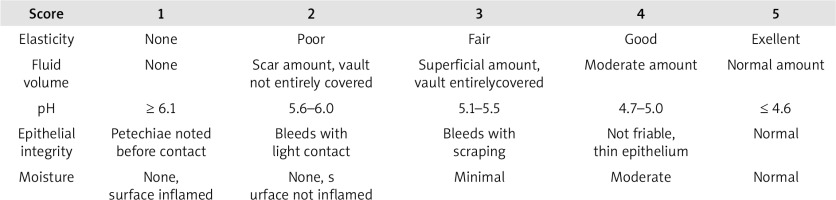
Also, the clinician evaluated the vaginal health index (VHI), a quantitative assessment of vaginal health, by considering local features of the epithelium such as elasticity, fluid volume, pH, epithelial integrity, and moisture (Fig. 2).
The epithelium was evaluated at baseline (VHI-0), one month after the beginning of treatment (VHI-1), and 2 months after the end of the 4th session (3.5 months after the beginning of therapy) (VHI-2).
Data were collected at the beginning of the treatment and at follow ups for each patient, as shown in Table 1.
Table 1
Data collection
Once data collection was done, we calculated the mean value for both NRS and VHI values at time 0, at one month after treatment beginning, and at 2 months after the last session.
Informed consent was obtained from all subjects involved in the study.
The study was conducted according to the guidelines of the Declaration of Helsinki.
Results
The study included 18 postmenopausal women treated externally and internally with Ladylift® non- ablative laser technology and evaluated at the beginning of the treatment, at one month, and at 2 months after the end of the 4th session follow-up.
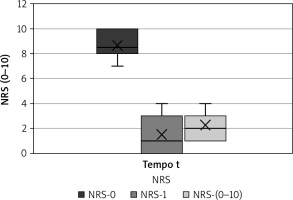
The numeric rating scale used to measure local pain had a mean value of 9, with lowest score 7 and highest score 10 before the procedure (NRS-0), a mean value of 2, with lowest score 0 and highest 4 at one month after treatment beginning (NRS-1), and a mean value of 2, with lowest score 1 and highest 5 at two months after ending the last session (Fig. 3).
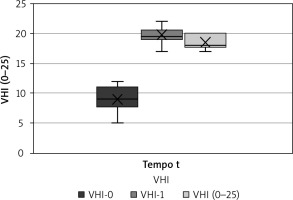
Complementary, VHI resulted in a mean value of 9 before treatment beginning, with lowest score 5 and highest score 12, a mean value of 20 at one month after treatment beginning, with lowest score 17 and highest score 22, and a mean value of 19 at two months after the end of the last session, with lowest score 17 and highest 20 (Fig. 4). We can see, therefore, that the patients undergoing laser therapy had evident benefit both from the point of view of pain and from that of vaginal health in general.
All women tolerated the therapy well without any adverse effects for the duration of treatment.
Discussion
Genitourinary syndrome of menopause affects up to 48% of pre-menopause women and up to 90% of menopausal women [1].
The symptoms are mainly genital (dryness, burning, irritation), sexual (dyspareunia, vestibulodynia, postcoital bleeding), and urinary (urgency, nocturia, recurrent urinary tract infection, incontinence) [1, 2, 5].
Few therapies have shown to be effective for PVD and GSM. Considering its multifactorial aetiology, the most effective approach for PVD is multimodal and multidisciplinary, with a biopsychosocial model tailored to each patient [7]. To date, psychological interventions, pelvic floor physical therapy, and vestibulectomy are the recommended empirically supported treatments for vulvodynia. Pharmacological treatments that may be beneficial include antinociceptive agents (lidocaine, capsaicin), anti-inflammatory agents (corticosteroids, interferon), neuromodulating medications (anticonvulsants and antidepressants), hormonal agents, and muscle relaxants (e.g. botulinum toxin). Nevertheless, these treatments all require further placebo-controlled study [18].
Laser therapy is a recently known alternative approach with few adverse effects and good outcomes for vaginal health and symptoms.
In a case series [12], women with either vestibulodynia or GSM underwent 3 sessions of fractional ablative CO2 laser treatment. No group differences were observed. However, 67.4% of women reported significant improvements in pain during intercourse, and these improvements were maintained at 4-month follow-up. In another double-blind, placebo-controlled, small RCT [19], women reported improvements in their intercourse pain after 12 sessions of laser therapy compared to those in the placebo group.
Traditionally, GSM symptoms have been treated with either non-hormonal or hormonal therapies.
Topical hormonal treatment is first-line therapy in GSM, but it has some side effects (thromboembolism, oestrogen-dependent gynaecological malignancies) that contraindicate its use in some women. Systemic oestrogen therapy is sometimes used; however, 10–20% of women may have residual symptoms even while taking systemic oestrogen [10, 20]. Non-hormonal therapies include water- or silicone-based vaginal lubricants, vaginal moisturizers, herbal remedies, soy products, and oestrogen agonists and antagonists (such as ospemifene) [15, 21, 22]. They may be used in women of any age in whom hormonal treatments are contraindicated. However, their real benefit remains controversial due to their short-term effect [10, 21].
The use of radiofrequency ablation of vaginal epithelium is currently being investigated as a non-hormonal treatment option. Many studies compared the efficacy of laser CO2 therapy to local hormonal therapy, proving its cost-effectiveness and safety and showing comparable outcomes in improving GSM symptoms [11–13].
In 2020 Pagano et al. [23] were the first to perform a histologic assessment of vaginal epithelium changes in patients affected by GSM treated with fractional CO2 laser therapy. Vulvar biopsies were taken before treatment and after 3 sessions. Differences in means before and after treatment were significant, with 93.3% of patients showing remodelling of vulvar connective tissue, 80% improvement in vulvar epithelium trophism, and 86.7% neovascularization.
Restored epithelium reduces symptoms of GSM not only by improving sexual satisfaction [23] and urinary stress incontinence, but also reducing pelvic organ prolapse (POP) [24] and provoked vulvodynia [12].
Both Sokol et al. and Behnia-Willison et al. [25, 26] showed long-term effects of laser therapy at one-year follow-up, with significant maintenance of patient satisfaction and improvement of symptoms.
These reports show that further studies with a larger population, various treatment protocols, and evaluation of fraction ablative CO2 laser treatment in different subgroups of PVD are needed to define which patients can benefit from this therapy.
The main difference of Ladylift® compared to other laser technologies is the use of a non-ablative laser wavelength of 1470 nm, which penetrates in depth, without causing ablative thermal injury on the surface of the mucosa, thus avoiding burns and scars.
The treatment protocol we present consists of 4 sessions of laser, 2 weeks apart, with a duration of a few minutes (4 minutes on average), performed without anaesthesia because it is completely painless. The study included 18 postmenopausal women with GSM symptoms and PVD treated both externally and internally with Ladylift®. The benefits tin terms of menopause symptoms were evaluated by pain perception, using the NRS, and by epithelium trophism evaluated with VHI.
Overall, the pain halved with a maximum NRS of “10” before treatment and maximum NRS of “5” at 2 months after the end of the last session. Similarly, VHI showed improvements after treatment with a mean value of 9 before treatment and a mean value of 19 two months after the end of the treatment. Nonetheless, VHI was subsequently dropped by one point of mean value between the evaluation at one month (mean value 20) and at 2 months after the end of the procedure (mean value 19). This is probably due to the few sessions to which we chose to refer our patients, therefore suggesting the need to perform more therapy sessions or the need to perform therapy for longer periods of time. To date, there is no given consensus regarding treatment protocol with session numbers, rates, and frequency of follow-up.
Finally, all women tolerated the therapy well without any adverse effects for the duration of treatment. This result supports the concept that Ladylift® non-ablative laser technology is safe and effective when improving GSM symptoms and vaginal health.
The strength of our study is based on the presence of a homogeneous groups of patients from the point of view of NRS and VHI (all patients reported a NRS score at time 0 between 7 and 10 and a VHI between 7 and 11), before therapy. Furthermore, patients had a similar age (50–58 years) and have been subjected to laser therapy for the same period with a very similar exposure per session (4 minutes).
The limitations are based on the presence of a small sample of patients and with limited observation in time (only 7 and 30 days after the end of treatment).
This should be considered as a preliminary study. It is always difficult to initially test a new therapy on a large sample of the population. However, we intend to expand the sample and perform long-term evaluation on the vaginal health in the future.
Conclusions
In this prospective study, we tested the effectiveness and safety of a new non-ablative solid-state laser, Ladylift®, on a sample of postmenopausal women with provoked vulvodynia and GSM. We observed an improvement in the VHI and a reduction in the NRS pain scale up to one month after the end of treatment. However, the beneficial effect tended to gradually decrease over time, suggesting the need to perform more therapy sessions for a longer period of time. This is a cohort study and further larger studies are needed to confirm our findings. Longer duration tests and on a larger sample are needed to understand the efficacy of the CO2 laser over time, allowing us also to standardize the number and the length of time between sessions to optimize their effectiveness.