Introduction
Leiomyosarcoma (LMS) of the vulva is a rare disease representing 1% of all primary vulvar neoplasms. Still, it is the most common type of vulvar sarcomas [1-3]. It occurs usually in the Bartholin's gland area, and its clinical picture is similar to that of bartholinitis which can be diagnostically challenging [4]. It is more common in middle-aged or older women [5]. Most of the described cases had been reported in Western countries, pointing to the conclusion of a possible etiopathogenetic link to lifestyle or genetic predisposition [5]. Leiomyosarcoma can arise from the smooth muscles, the walls of blood vessels, rough ligaments, and erector–pili muscles [6-8].
Isolated cases and limited series have been described in research literature [7, 9-12], leading to a lack of consensus on both surgical treatment and adjuvant radiotherapy and/or chemotherapy.
We present a case of a 73-year old patient hospitalised for surgical treatment due to a painful fast-growing formation in the symphysis area.
Case report
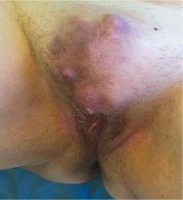
A 73-year old patient was admitted to the clinic with a formation with irregular margins in the symphysis area, with dimensions of 7/5 cm and satellite nodules 20 mm in diameter on average (Fig. 1).
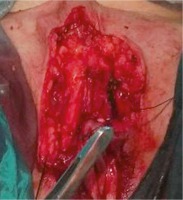
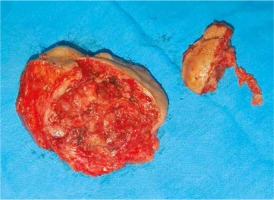
Arterial hypertension and previous cholecystectomy were reported in anamnesis. The formation has been present for several years but in the last 3 months it has started to grow rapidly (it has doubled in size), accompanied by pain. Radiography of the lungs, abdominal ultrasound, and CT of the small pelvis did not show any abnormalities. After standard preoperative preparation, a wide local excision of the described formation was performed (Figs. 2 and 3).
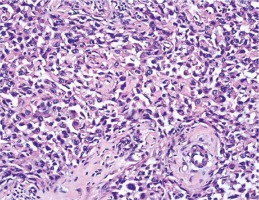
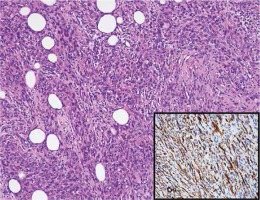
Microscopic examination showed subcutaneous infiltration of an overtly malignant mesenchymal tumour. Part of the parenchyma was represented by elongated cells with pronounced cellular and nuclear atypia, forming chaotically intertwined bundles, areas of tumour necrosis and single mitotic figures. In many fields, the cells were markedly rhabdoid in appearance, large with abundant eosinophilic cytoplasm, and excentric polymorphic nuclei, which aroused suspicion of rhabdomyosarcoma. We diagnosed leiomyosarcoma, based on the morphological and IHC testing: SMA /+/, S100 /–/ and MyoD1 /–/ (Figs. 4 and 5).
Fig. 4
Classical picture of leiomyosarcoma manifested by elongated cells with moderate nuclear polymorphism and abundant eosinophilic cytoplasm. XE × 0.25; cytoplasmic expression for SMA (insertion × 0.40)
The patient was discharged on day 2 postoperatively and referred for radiotherapy.
Discussion
The incidence of vulvar sarcomas varies between 1.5% and 5% of all vulvar neoplasms, with LMS being the most frequent histological type [1, 13]. Other vulvar sarcomas include embryonal and alveolar rhabdomyosarcoma, epithelioid sarcoma, alveolar soft part sarcoma, and other rare sarcomas [4, 14].
Isolated cases and limited series of LMS cases have been described in literature and the largest series (25 cases) was reported by Aartsen and Albus-Lutter [10].
LMS is most commonly localized to the labia majora, the Bartholin gland area, clitoris and labia minora [15]. LMS can affect all age groups and most often involves perimenopausal women [10]. The youngest patient reported with the disease is a 14-year old [11]; some cases of leiomyosarcoma during pregnancy have been described as well [12]. Most of the reported cases had been identified in Western countries, indicating a potential association to lifestyle or genetic predisposition [5]. A possible involvement of chronic inflammation to LMS has been discussed [16]. LMS cases associated to lichen sclerosus have been reported [17].
Clinically, this tumour usually presents itself as an asymptomatic solid formation 1.5 to 16 cm in size, and may rarely be accompanied by bleeding or urinary problems [4, 7]. Bartholin cyst, infectious granuloma, fibroma, lipoma, or myomas should be considered in the differential diagnosis [18]. Improper diagnosis leads to inadequate treatment. Accurate diagnosis is made histologically. Unfortunately, there is no single morphological feature that clearly separates benign from malignant genital smooth muscle tumours. The most commonly used modified scheme for histological diagnosistoday is that of the Stanford researchers [19]. They introduced tumour cell necrosis coagulation as an additional feature to hitherto used mitotic index [20], and cellular atypism [21]. The latest 2014 WHO classification of tumours of the female reproductive organs also indicates size over 5 cm and invasive growth [14, 22, 23]. Immunohistochemical (IHC) testing is important in diagnosis as the smooth muscle nature of the tumour is not always obvious, as it was in our case. Desmin, smooth muscle actin and k-caldesmon [14] are most often positive.
There are no definitive therapeutic algorithms due to the rarity of the disease. The management is surgical treatment and the entire tumour must be removed with histologically verified clean resection margins, followed by radiation therapy which is not necessary in low-grade LMS [24, 25]. The administration of chemotherapy is not quite clear yet; it is considered that it may reduce the risk of recurrence or metastasis [26]. Radical tumour removal and the absence of metastases provide good prognosis [24, 26]. Some authors believe that lymphatic dissection should be performed [1, 6]. LMS has the ability to metastasize hematogenously and to recur [8].
In our case, the patient was significantly older than the average age reported for the disease and had a pronounced pain symptom, which we attribute to the necrotic changes caused by the rapid tumour growth. We performed extensive local excision followed by radiation therapy. Three months later, the patient is well with no evidence of recurrence.
Conclusions
Vulvar tumours cannot always be macroscopically diagnosed as malignant or benign. In many cases, differentiation is difficult even in histological examination, which is the main diagnostic method. Malignancy and histogenesis of these tumours are not always obvious. This makes them unsuitable for express histological testing (freeze-drying of tissue) during surgical treatment. Accurate diagnosis allows adequate disease management and treatment.