Introduction
The treatment of choice in early stage non-small cell lung carcinomas (NSCLC) is surgery [1–3]. Only one randomized trial that was published in 1995 has compared lobectomy with sublobar resection in terms of overall survival [1]. JCOG0802/WJOG4607L as a randomized trial showed the superiority of segmentectomy over lobectomy in terms of overall survival in peripheral stage 1A1-1A2 NSCLC [3]. Moreover, it showed that segmentectomy is superior to lobectomy in terms of 5-year overall survival in non-small cell lung carcinomas ≤ 2 cm. Similarly, Altorki et al. conducted a similar study and found that, in patients with peripheral NSCLC with a tumor size of 2 cm or less and no lymph node metastasis, sublobar resection was not inferior to lobectomy in terms of disease-free survival [4].
The results of these studies suggest that, for patients diagnosed with clinical stage IA peripheral NSCLC and small-sized tumors (≤ 2 cm with a consolidation-to-tumor ratio > 0.5), segmentectomy should be considered as the preferred surgical procedure over lobectomy [3, 4].
Wedge and segmentectomy, as sublobar resection techniques, are viable options for patients diagnosed with early-stage non-small cell lung carcinoma who are preoperatively identified as high-risk candidates. These procedures are particularly suitable for individuals who have comorbidities, limited respiratory reserve, or small lesion sizes [5, 6].
While conventional adjuvant treatment is typically not advised for patients with stage 1 non-small cell lung carcinoma, it is important to note that standard mediastinal lymph node dissection is recommended when sublobar resection is performed. Furthermore, administering chemotherapy or radiotherapy as adjuvant treatment based on the pathological findings of the excised lymph nodes has demonstrated substantial survival benefits [7].
The term “completion lobectomy” refers to a safe surgical technique involving the anatomical removal of the remaining lung tissue. This procedure is typically performed following diagnostic wedge or segmentectomy resections, or when pathological data are required [8–10]. However, the role of completion lobectomy in the nodal upstaging has not been studied.
Aim
The primary endpoint of the study was to determine whether completion lobectomy in patients with T1a–c NSCLC results in similar or higher rates of nodal upstaging compared to initial lobectomy or sublobar resection. The secondary endpoint was to assess the impact of resection type (lobectomy, sublobar resection, or completion lobectomy) on overall survival in this patient population.
Material and methods
Ethics committee approval was received for our study (date: 21/10/2022, number: E-83045809-604.01.01-514113). Patients who were operated on in our clinic for non-small cell lung carcinoma between January 2001 and January 2023 were retrospectively analyzed. Patients with tumor diameter > 3 cm according to the final pathology report were excluded from the study. A total of 477 patients were analyzed. Forty-five patients with a tumor size of ≤ 3 cm but who underwent bilobectomy or pneumonectomy were excluded from the study. Patients with tumor size ≤ 3 cm were divided into subgroups according to completion lobectomy, sublobar resection, or lobar resection: 117 patients (87 males/30 females; mean age: 62 ±20.8 years) who underwent completion lobectomy; 45 patients (26 males/19 females; mean age: 63 ±8.5 years) who underwent sublobar resection; 270 patients (202 males/68 female; mean age: 61 ±10.1) who underwent lobar resection.
Recorded and analyzed parameters included age, sex, clinical data (comorbidity, diabetes, tuberculosis, body mass index, cardiac risk index, pulmonary risk index, Charlson comorbidity risk index, cigarette pack-years, complications, postoperative hospital stay, and need for intensive care), respiratory parameters (FVC, FEV1, %FVC, %FEV1, FEV1/FVC, DLCO, DLCO%, DLCO/VA, %DLCO/VA, ppoFEV1, ppoDLCO, PaO2, PaCO2), preoperative laboratory values (albumin, CRP, LDH, leukocytes, lymphocytes, monocytes, neutrophil, hemoglobin), PET/CT data (tumor SUVmax and lymph node SUVmax value), surgical data (VATS/thoracotomy, tumor side, tumor location), pathological characteristics of the tumor (pathological diagnosis, N0, N1, N2, tumor diameter, T stage, 8th TNM stage, pleural invasion, perineural invasion, lymphatic invasion, vessel invasion, presence of spread through air spaces [STAS]) and follow-up data. Mean follow-up time was 89 months (6–189 months).
The groups that underwent completion lobectomy, sublobar resection and lobar resection were divided into subgroups according to tumor size in the range of 0–2 cm and in the range of 2–3 cm. These subgroups were evaluated for N0, N1, N2 status and overall survival data. The patients with cT1a-T1b underwent intentional videothoracoscopic segmentectomy (multiportal or uniportal), whereas patients with cT1c underwent segmentectomy when they had limited lung function (ppoFEV1 < 40% and/or ppoDLCO < 40%). Patients with postoperatively disclosed N2 disease or R1 disease underwent completion lobectomy through the same incision.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA). The normality of continuous variables was assessed using the Kolmogorov-Smirnov test. Continuous variables with normal distribution were expressed as mean ± standard deviation, while non-normally distributed variables were expressed as median (minimum-maximum). Comparisons among the three groups were performed using one-way ANOVA for normally distributed continuous variables and the Kruskal-Wallis test for non-normally distributed continuous variables. When a statistically significant difference was found, post-hoc analyses (Tukey or Dunn test) were conducted. Categorical variables were compared using the χ2 test or Fisher’s exact test when the expected cell frequency was low.
Survival analyses were performed using the Kaplan-Meier method, and differences between groups were assessed using the log-rank test. Multivariate survival analyses were conducted using the Cox proportional hazards regression model. A p-value of < 0.05 was considered statistically significant.
Results
There was no statistically significant difference between the groups in terms of age and other demographic parameters except sex (Table I).
Table I
Univariate analysis of demographic characteristics, respiratory function tests, basic biochemical parameters, and clinical and pathological data
Tumor SUVmax and lymph node SUVmax values were statistically significantly higher in the lobar resection group compared to those of Group 1 and Group 2 (p < 0.001, p < 0.001, respectively) (Table I). Hospital stay and amount of drainage were statistically significantly lower in the sublobar resection groups (p = 0.015, p = 0.026, respectively) (Table I).
The presence of N1 was statistically significantly higher in the lobectomy group(p < 0.001) (Table I). VATS resection rate and presence of N0 were statistically significantly higher in the sublobar resection group (Group 2) (p < 0.001, p < 0.001, respectively) (Table I). Presence of perineural invasion, lymphatic invasion, and vascular invasion was statistically significantly less frequent in the sublobar resection group (p = 0.001, p = 0.032, p = 0.004, respectively) (Table I).
There was no statistically significant difference in survival in terms of performed operation (i.e., segmentectomy vs lobectomy vs completion lobectomy) (p = 0.164) (Figures 1, 2; Table II).
Table II
Univariate analysis of survival outcomes across study groups
Figure 1
Evaluation of survival data between groups by Kaplan-Meier analysis. There was no statistically significant difference in survival between the 3 groups
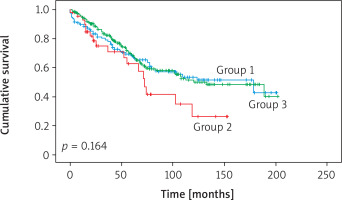
Figure 2
Evaluation of survival data between groups by Cox regression analysis. There was a statistically significant difference in survival between the 3 groups (Group 1: completion lobectomy, Group 2: sublobar resection, Group 3: lobar resection)
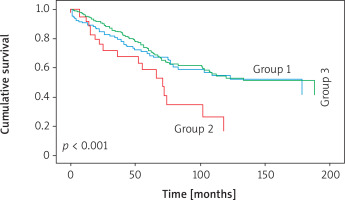
Nodal status, perineural invasion, and stage were found to be independently associated with survival (p = 0.002 and p = 0.02 respectively; Table III).
Table III
Results of dependent variables in Cox multivariable analysis
No statistically significant differences were observed among the three groups in terms of nodal involvement (Table IV). However, when the patients with T1ab tumors were evaluated, lobectomy provided better survival compared to those who had lobar resection or completion lobectomy (p = 0.037) (Figure 3 A; Table II).
Table IV
Evaluation of 3 groups for N status in patients with T1a-b and T1c
Figure 3
A – Survival of patients with T1ab tumors. Statistically significantly worse survival was observed in Group 2 (sublobar resection). B – Survival curves of patients with T1c tumor. There was no statistically significant difference between the groups
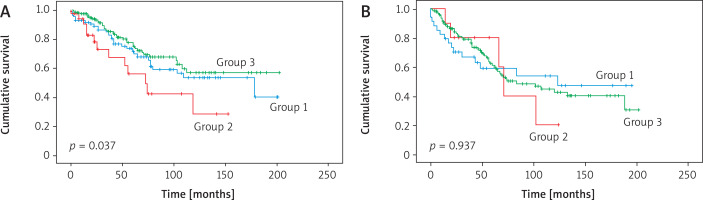
In the second subgroup analysis, while the N0 rate was statistically significantly higher in the group with 2–3 cm sublobar resection, the N1 status was statistically significantly less frequent (p = 0.012, p = 0.018, respectively; Table IV). There was no statistically significant difference in survival in patients with T1c NSCLC (p = 0.937) (Figure 3 B; Table II).
Discussion
In our study, for T1a-bN0M0 NSCLC patients there was a statistically significant difference in nodal upstaging between patients who underwent lobectomy or sublobar resections. N1 metastases were identified more frequently in patients who underwent lobectomy.
The observed statistically significant prevalence of female sex within the patient group undergoing sublobar resection, and its association with fewer complications and improved survival in this group of female patients, might explain the higher incidence of segmentectomy in women [11, 12]. In our study, the findings that the patients had shorter hospital stay were similar to the literature [13, 14]. The elevated values of tumor SUVmax and lymph node SUVmax in PET/CT results likely influenced the decision to perform lobar resections more frequently in these patients within our clinical practice.
In the context of pathological and surgical data, the sublobar resection group displayed a lower tumor stage and a higher incidence of N0 status. Additionally, the occurrence of perineural, lymphatic, and vascular invasion was relatively low. These findings suggest that in patients with less aggressive pathological characteristics, minimally invasive sublobar resection is favored, and the need for additional lobectomy is less common [12, 14].
No statistically significant difference was observed in terms of overall survival between the groups of patients who underwent lobectomy or sublobar resection. However, sublobar resection was independently associated with statistically significantly worse survival with multivariate analysis. Furthermore, N status, TNM stage, and perineural invasion were individually found to be statistically significant independent factors associated with overall survival. Based on these findings, it is advisable to recommend the significance of completion lobectomy for up-staging concerning “T” and “N” factors [15].
Within the subgroup of tumors measuring 0–2 cm (i.e. T1a-b), a statistically significant decline in survival was observed in the sublobar resection group, with no discernible difference in N status. This underscores the advantage of considering a completion lobectomy in cases where there is an up-staging in the “N” category [16]. However, the absence of a survival difference in the 2–3 cm subgroup, coupled with the higher frequency of N0 and the lower occurrence of N1 in the sublobar resection group, suggests that sublobar resection may be a suitable option for patients with tumors in the 2–3 cm range with a low incidence of upstaging [17].
The study has some limitations. It was a retrospective study conducted in a single institution. Although the demographic parameters were well balanced between the study groups, our analysis does not fully address all selection biases in patient selection. Similarly, the lack of recurrence data limits the strength of this evaluation.
Luo et al. conducted an analysis using the National Cancer Database (NCDB), including more than 123,000 patients, and concluded that survival in patients with occult pN1 and pN2 disease discovered after segmentectomy was at least equivalent to that of lobectomy [18]. However, their study did not compare outcomes in patients who underwent completion lobectomy. In our own study, we found that occult N1 and N2 disease was more frequently identified in patients who initially underwent lobectomy compared to those who had segmentectomy. Furthermore, we observed that performing a completion lobectomy in these patients did not confer any additional survival benefit. Moreover, patients with T1a and T1b tumors lobectomy showed worse survival compared to segmentectomy, which is in line with the JCOG 0802 study [3]. In addition, it has been reported that segmentectomy did not provide a better prognosis in cT1cN0M0 NSCLC patients [19]. Similarly, among patients with cT1cN0M0 disease, we did not observe any statistically significant difference in survival between those who underwent lobectomy and those who had sublobar resection.
On the other hand, Iwai and colleagues reported that lobectomy was more frequently performed for T1N2 lung cancer compared with segmentectomy [20]. They found that lobectomy offered a significant survival advantage over segmentectomy. We routinely performed mediastinal staging in patients with clinical N1 or N2 disease using EBUS-TBNA or mediastinoscopy. This likely contributed to the low number of patients with pathologic N2 disease in our series.
Conclusions
Lobectomy is associated with higher upstaging in cT1N0M0 non-small cell lung cancer patients. Completion lobectomy is safe and feasible in T1 non-small cell lung carcinomas when needed if up-staging occurs in terms of N status. However, completion lobectomy did not seem to offer a survival advantage in patients with cT1a-cN0. Moreover, lobectomy seems to be associated with worse survival in patients with smaller (< 2 cm) tumors. However, there is a need for randomized trials in order to obtain better evidence regarding the possible benefits of lobectomy in cT1cN0M0 patients.






