Summary
The coexistence of atrial fibrillation and chronic kidney disease increases the risk of thromboembolic complications, including ischemic stroke. One method of prevention is percutaneous left atrial appendage occlusion (LAAO). Currently, there are few data evaluating this method in patients with impaired renal function. Our study included a large group of patients with renal failure. The study aimed to evaluate the safety, efficacy and long-term outcomes of LAAO performed in patients with non-valvular AF and chronic kidney disease not only in the advanced stage of the disease, but also in mild cases. Our registry included LAAO procedures using also a newer generation of occluders. The study generated interesting results regarding a significant reduction in the number of thromboembolic events while reducing the risk of serious bleeding complications, which may indicate new alternatives in daily clinical practice in this group of patients.
Introduction
Atrial fibrillation (AF) is the most common supraventricular cardiac arrhythmia in the general population, and its prevalence increases with both age and comorbidities [1]. It is estimated that 3% of people over 20, 6% of people over 65 and up to 9% of people over 80 suffer from AF [2], and a 2.3-fold rise is expected [3]. One of the independent AF risk factors is chronic kidney disease (CKD). According to reports, the prevalence of CKD in developed countries is as high as 8.6% in men and 9.6% in women and is associated with a reduced life expectancy compared to the general population, mainly due to a significantly increased risk of developing cardiovascular diseases [4]. In CKD patients, AF incidence increases as renal functions worsen, reaching nearly 15% in dialysis patients [5]. The most serious complication of AF is an ischemic stroke, whereas the coexistence of AF and CKD leads to a significant increase in the risk of both thromboembolic complications and hemorrhagic incidents. To reduce the risk of thromboembolic events and improve the prognosis in AF patients, the use of chronic anticoagulation therapy is crucial. However, a significant percentage of patients have relative, and nearly 2% of them absolute, contraindications to their use, which are much more common in AF patients with CKD compared to other AF patients [6]. Percutaneous left atrial appendage occlusion (LAAO) is a treatment alternative whose numerous benefits have been confirmed by randomized trials and prospective analyses [7, 8]. It is based on the assumption that the left atrial appendage (LAA) is the site of nearly 91% of all thrombi in non-valvular AF [9]. Continuing chronic anticoagulation therapy after a minimally invasive LAAO procedure is typically not necessary in most patients [10]. This brings significant benefits for patients with AF and CKD, who are already at a very high risk of hemorrhagic incidents as it is.
Aim
This study aimed to evaluate the safety, efficacy and the long-term outcomes of LAAO performed in patients with CKD and non-valvular AF.
Material and methods
The study was a single-center, prospective observational study. A total of 272 consecutive patients with non-valvular AF (paroxysmal, persistent, or permanent) who underwent LAAO between 2009 and 2019 were included in the study.
Study population
All included patients had indications for stroke prevention in the course of AF based on the European Society of Cardiology (ESC) class I recommendations (men with a CHA2DS2-VASc score ≥ 2 and women with score ≥ 3), with contraindications to oral anticoagulant treatment [3].
Based on pre-procedure laboratory tests, eGFR was calculated in each patient, based on the MDRD formula, in the following way: eGFR (ml /min/1.73 m2) = 175 × {} Skreat}–1.154 × [age in years]–0.203 × [0.742–1.154 × [0.742 women] × [1.21 dark race].
In the Kidney Disease Improving Global Outcomes (KDIGO) guidelines [11], CKD is defined as eGFR less than 60 ml/min/1.73 m2. Additionally, patients were classified according to the different disease stages using the following grades: G1 ≥ 90 ml/min/1.73 m2; G2 60 to 89 ml/min/1.73 m2; G3a 45 to 59 ml/min/1.73 m2; G3b 30 to 44 ml/min/1.73 m2; G4 15 to 29 ml/min/1.73 m2; G5 < 15 ml/min/1.73 m2 or undergoing dialysis treatment.
Two groups of patients were distinguished based on the eGFR value. The non-CKD group included 167 (61.4%) patients, 39 of whom (14.3%) were stage G1 patients (9 women, 30 men) and 128 of whom (47.1%) were stage G2 patients (56 women, 72 men). The CKD group consisted of 105 (38.6%) patients diagnosed with CKD, with 50 (18.4%) stage G3a patients (24 women and 26 men), 37 (13.6%) stage G3b patients (21 women and 16 men), 17 (6.3%) stage G4 patients (11 women and 6 men), as well as 1 male patient at stage G5 (0.4%).
The expected annual rates of major bleeding and stroke were calculated from the individual HAS-BLED [12] and CHA2DS2-VASc score, respectively [13].
Since renal impairment is a strong predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation and is not included in the CHA2DS2-VASc score, the risk of ischemic stroke was also assessed for each group using the R2CHADS2 score, which includes a creatinine clearance value [14].
Before the LAAO procedure
All patients underwent transesophageal echocardiography (TEE) before the procedure to examine the morphology of their LAA. Utmost attention was also paid to assessing the presence of thromboembolic material, as well as factors increasing the risk of stroke in AF, such as sediment or spontaneously contrasting blood.
The loading doses of acetylsalicylic acid and clopidogrel were administered before the procedure in patients who had not previously received these drugs and if there were no contraindications to using them.
LAAO procedure
LAAO procedures were performed under sedation (administration of midazolam) or general anaesthesia. In the first stage, after obtaining vascular access (via the right femoral vein), a transseptal puncture sheath was inserted. Using TEE (2D or 3D) and fluoroscopy, the interatrial septum was punctured in the lower posterior part of the fossa ovalis, which was followed by selective angiography in the LAA. Once the catheter was positioned at the appropriate depth in the neck of the LAA, an occluder was inserted. The occluder was selected based on measurements conducted using TEE and LAA angiography. Before releasing the occluder, a traction test was performed to confirm that it was stable. After the catheter was removed from the groin, a hemostatic suture was applied.
Devices
During the procedures, the Amplatzer Cardiac Plug (ACP) was used in 51 (18.7%) patients (St. Jude Medical, Saint Paul, Minnesota), the Amplatzer Amulet (Abbott Vascular, Santa Clara, USA) in 205 (75.4%) subjects, whereas the Watchman (Boston Scientific, Marlborough, USA) was used in 13 (4.8%) patients.
After the LAAO procedure
After the procedure, dual antiplatelet therapy consisting of 75 mg acetylsalicylic acid and 75 mg clopidogrel was recommended to the patients until their first follow-up visit 4–6 weeks afterwards.
TEE or computed tomography (CT) was performed during their next visit to verify the occluder’s position in the LAA, as well as to check for any residual leaks around the occluder and any potential thrombi related to its presence. If no thrombi were discovered in the left atrium and around the occluder, clopidogrel therapy was discontinued; with no contraindications, treatment using 75 mg doses of acetylsalicylic acid was recommended to be continued indefinitely. During the follow-up visits, the clinical condition of the patients was assessed, and data on adverse effects such as ischemic and hemorrhagic stroke, as well as the occurrence of major bleeding, were collected. Based on the ISTH criteria, major bleeding was defined as symptomatic and [15]: fatal bleeding and/or bleeding in a critical area or organ, such as intracranial, intramedullary, intraocular, retroperitoneal, intraarticular, or pericardial or intramuscular, with tightness syndrome and/or bleeding causing the hemoglobin level to drop by 20 g/l (1.24 mmol/l) or more or leading to the transfusion of two or more units of whole blood or red blood cells.
Follow-up
The mean follow-up in the study population was 25.56 ±17.9 months. The mean follow-up in the non-CKD group was 27.53 ±18.7 months and 22.3 ±15.7 in the CKD group (p = 6.017).
Ethics
The study was conducted in accordance with the principles of the Helsinki Declaration. All patients gave their written consent to the procedure and the analysis of the scientific data. The indications for the procedure were in line with the current ESC guidelines. Moreover, due to the observational nature of the study, the consent of the Ethics Committee was not required.
Statistical analysis
Statistical calculations were performed using Jamovi 1.2 and R Core 3.6 software.
Qualitative variables are presented as counts with percentages, while quantitative variables are presented as a mean and standard deviation. The distribution was evaluated using the Shapiro-Wilk test. Depending on the nature of the variables being compared, either the χ2, Mann-Whitney U or Kruskal-Wallis test was used to show the significant differences between the two groups. A level of significance at p < 0.05 was considered statistically significant. A Kaplan-Meier curve was used to estimate the survival function.
Results
The mean serum creatinine levels were 81.6 ±15.0 μmol/l in the non-CKD and 146 ±59.5 μmol/l in the CKD group (p < 0.001). Patients in the CKD group were significantly older than those in the non-CKD group. The mean age in the non-CKD group was 70.9 ±9.08 years, while in the CKD group it was 75.6 ±7.52 years (p < 0.001). Patients in the non-CKD group had a significantly lower risk of ischemic stroke estimated by the CHA2DS2-VASc score (3.73 ±1.42 points) compared to patients in the CKD group (4.86 ±1.27; p < 0.001). Bleeding risk assessment based on the HAS-BLED score also showed a significantly lower risk of bleeding in the non-CKD group (2.89 ±0.703) compared to the CKD group (3.20 ±0.837; p = 0.003). The risk of stroke estimated using the R2CHADS2 score was 2.19 ±1.30 points for the non-CKD group and 4.34 ±1.48 points for the CKD group (p < 0.001). Statistically significant differences were found between the groups. Tables I and II show the detailed characteristics of both groups.
Table I
Patient characteristics – demographics
Table II
Patient characteristics – selected laboratory and echocardiographic parameters
A successful LAAO procedure was performed in the case of 269 (98.9%) study population patients, 165 (99.4%) from the non-CKD group and 103 (98%) from the CKD group. The attempt to close the LAA proved unsuccessful in 3 (1.1%) cases.
Additionally, the procedure was performed in 7 (2.6%) patients with an existing thrombus in the left atrial appendage. In 4 (1.5%) cases, the Sentinel Cerebral Protection System (Boston Scientific, Marlborough, Massachusetts) was used to protect the cerebral circulation as well.
A long-term follow-up visit was carried out in all 272 (100%) included patients.
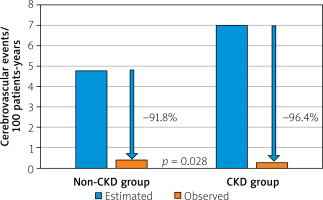
Seven (2.6%) patients suffered from an ischemic stroke, including 2 (1.9%) patients in the CKD group and 5 (3.0%) patients in the non-CKD group (p = 0.581). The risk of ischemic stroke after LAAO was 0.39/100 patient-years (PY) in the non-CKD group and 0.25/100 PY in the CKD group. Meanwhile, the predicted mean risk of thromboembolic events in patients with similar CHA2DS2-VASc scores but without anticoagulation was 4.79/100 PY in the non-CKD group and 7.00/100 PY in the CKD group on average. Based on the analysis of these data, there was a significant reduction in the estimated risk of thromboembolic events, including ischemic stroke in both groups of patients undergoing LAAO (by an average of 91.8% in the non-CKD group and an average of 96.4% in the CKD group – whereas the beneficial effect of LAAO was stronger in the CKD group) (Figure 1).
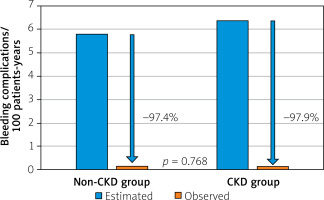
There was also a significant reduction in major hemorrhagic incidents in both groups compared to their predicted risk based on the HAS-BLED scale. During the long-term follow-up a hemorrhagic stroke occurred in 1 patient from the non-CKD group (0.6%), while major bleeding occurred in 1 patient from the non-CKD group (0.6%) and 1 subject from the CKD group (1.0%). There was no statistically significant difference in the number of major bleeding incidents (p = 0.740) or hemorrhagic strokes (p = 0.427) between the two groups studied. The predicted risk of hemorrhagic complications in patients after the procedure in the non-CKD group and the CKD group was 0.15/100 PY and 0.13/100 PY, respectively. Meanwhile, during VKA therapy based on a comparable HAS-BLED score, the risk of hemorrhagic complications for patients in the non-CKD group was 5.81/100 PY, compared to 6.40/100 PY in the CKD group. The analysis of the above data shows a reduction in hemorrhagic complications of 97.4% in the non-CKD group and 97.9% in the CKD group (Figure 2).
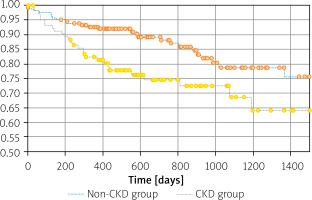
Fifty-four (19.8%) patients died during the entire follow-up period – 26 (15.5%) in the non-CKD group and 28 (26.7%) in the CKD group (p = 0.025). The Kaplan-Meier curves demonstrated significantly higher mortality in the CKD compared to the non-CKD group (p = 0.008). The unfavorable effect of CKD on mortality was already visible in the early postoperative period (Figure 3).
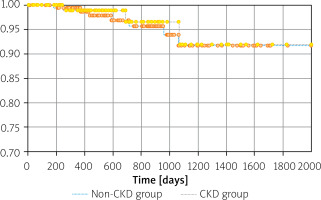
Based on the analysis of the adverse event-free survival curves, there was no statistically significant difference in the incidence of hemorrhagic and thromboembolic events between the analyzed groups (p = 0.785) (Figure 4).
Discussion
This prospective study was conducted in 272 consecutive LAAO patients with non-valvular AF and CKD. The following occluders were used: Cardiac Plug, Amulet and Watchman. The procedure was successful in 98% of CKD patients, which indicates high effectiveness in this group of patients. Moreover, the occurrence of adverse events was assessed. There was a significant reduction in the incidence of thromboembolic events, while reducing the risk of serious bleeding complications after LAAO in both groups of patients.
Success of LAAO procedure
This study confirms that the LAAO procedure is an effective therapeutic option for patients with CKD. Early use of LAAO was associated with low surgical success rate (about 90%) [16]. Advances in invasive cardiology, the experience of operators and the growing knowledge of the procedures have resulted in a significant increase in the success of the LAAO procedure. This is confirmed by our study, because the procedure was successful in 98% of patients with CKD. Our analysis was conducted on the results obtained from patients starting already with those with CKD stage G3a. Thus, procedural success is at a similarly high level at all disease stages. In a recently published report by Genovesi et al. [17], which involved 92 patients undergoing hemodialysis, the procedure had a success rate of 100%, suggesting that even advanced CKD is not a contraindication to its performance. It should be noted that in studies evaluating CKD patients undergoing LAAO published so far, patients with echocardiographically confirmed thrombus in the LAA were excluded. Our study population included 7 patients with LAA thrombus despite oral anticoagulation therapy who underwent LAAO. The procedure’s success rate in this group of patients was 100% and without thromboembolic events.
Efficacy in reducing thromboembolic events
Patients with CKD suffer from an increased risk of thromboembolic complications [18], which was also observed in our population, as 23.5% of patients with CKD suffered from an ischemic stroke before LAAO. However, despite a significantly higher risk of stroke as expressed in the CHA2DS2-VASc score in patients with CKD (starting from the moderate stage of the disease) compared to the non-CKD group, we observed a low and comparable percentage of such incidence during the long-term follow-up after a successful LAAO procedure in both groups. Furthermore, we obtained a 96.4% reduction in the risk of ischemic stroke in the CKD group and a 91.8% reduction in the non-CKD group compared to the estimated risk according to data reported by Lip et al. [13]. The higher reduction in the frequency of ischemic stroke observed in our study, compared to the reduction enabled by VKAs – which according to published data is up to 60% [19] – suggests that in the prevention of thromboembolic complications, LAAO may be superior to VKA therapy; however, further detailed research is required to support this hypothesis. Nonetheless, the above makes it possible to conclude that LAAO is an effective therapeutic option, and sometimes, when oral anticoagulation is contraindicated, it may be the only option available – especially in the case of patients with severe CKD.
Efficacy in reducing major hemorrhagic events
We also observed a reduction in the frequency of hemorrhagic complications compared to VKA therapy by as much as 97.9% in the CKD group, as well as by 97.4% in the non-CKD group in relation to the estimated risk calculated based on the HAS-BLED scale [12]. Therefore, it should be emphasized that LAAO shows a significant advantage over VKA in regard to the reduction of hemorrhagic complications, which is particularly vital in such a high-risk group as patients with CKD. Moreover, due to the exclusion of patients with eGFR < 30 ml/min/1.732 (eGFR < 15 ml/min/1.732 in the case of apixaban) [20–23] from randomized studies on the safety and efficacy of NOACs, there are still no data on the possibility of their administration in such patients. Thus, we hypothesized that LAAO might also have an advantage over NOAC in patients with severe CKD. Similar findings were reported by Brockmeyer et al. [24]; however, the population of this study was significantly smaller, as it comprised 146 patients, among whom 81 subjects had CKD. The multi-center Kefer et al. [25] and Luani et al. [26] registries also confirm the effectiveness of LAAO in the prevention of thromboembolic events with the simultaneous reduction of bleeding risk in patients with CKD. The first study included 1014 patients, 375 of whom were diagnosed with CKD. A significant reduction in both stroke and bleeding rates in patients with CKD was obtained compared to the predicted annual risk. However, this registry included LAAO procedures using only an older generation of occluder, the Amplatzer Cardiac Plug (ACP) (St. Jude Medical, Saint Paul, Minnesota). Our registry used newer generations of devices with increased stability and additional modifications to minimize the risk of device-related thrombosis (DRT) and less oversizing of the device [27]. Similarly, the study by Luani et al. [26], supports the above conclusions regarding the high efficacy of LAAO in stroke prevention in CKD patients; however, these data are based only on WATCHMAN occluders (Boston Scientific, Marlborough, USA). Therefore, based on our study, which applied the Amplatzer Cardiac Plug (ACP) (St. Jude Medical, Saint Paul, Minnesota), the Amplatzer Amulet (Abbott Vascular, Santa Clara, USA) and the Watchman (Boston Scientific, Marlborough, USA), it can be concluded that both the treatment success and long-term effectiveness are at a comparably high level, regardless of the type of occluder used.
Mortality rate
During the long-term follow-up, there was a significantly higher mortality rate in the CKD group (26.7% vs. 15.6%). It may be partially explained by the fact that, compared to non-CKD patients, patients with CKD were significantly older and had more comorbidities, especially cardiovascular diseases. Furthermore, as previous studies have shown, CKD occurring on its own is already associated with reduced life expectancy compared to the general population [28]. However, it should be noted that the incidence of ischemic stroke and hemorrhagic complications was comparable in both groups, and therefore the prognosis is the same regardless of CKD comorbidity.
Limitations
This study was not randomized, as patients were included in each group based on renal function (eGFR). Among patients with CKD only 1 patient was undergoing dialysis. This creates a heterogeneous group that may influence the results. Stroke diagnosis was based on patient-provided documentation, and patients did not have routine follow-up examinations, such as MRI or central nervous system CT scans; thus clinically silent strokes may not have been diagnosed.
Conclusions
The LAAO procedure is an effective method for preventing a stroke in AF patients with coexisting CKD. Considering the significant reduction in thromboembolic events, with a simultaneous reduction in the risk of major bleeding complications, it provides a safe and effective alternative – and often the only therapeutic option available for patients with CKD.