Introduction
Although poorly understood, metastasis is the defining characteristic of cancer and the primary cause of death for > 90% of cancer patients [1, 2].
Recent emphasis on oligometastatic disease (1–5 metastatic lesions) has been focused on the treatment plan for metastases [3, 4]. The aggressive local treatment of oligometastases, such as resection, stereotactic body radiotherapy (SBRT), radiofrequency ablation [5], and cryoablation [6] – alone or in conjunction with systemic chemotherapy – has been the topic of study [3, 4]. The adrenal glands (AGM) are the fourth most common site of metastases in malignant disease, with a reported prevalence of 9–27% [7].
With the advent of SBRT, which delivers a high dosage in a limited number of fractions with a high degree of conformity, it is now possible to deliver ablative doses, thereby expanding the role of radiation therapy. Stereotactic body radiotherapy is a prospective alternative therapy for metastatic lesions because it is noninvasive, effective, and tolerable [8]. Stereotactic body radiotherapy can accomplish durable disease control and even cure in certain patients [9, 10].
Metastases to the adrenal gland can originate from the lung (39%), breast (35%), gastrointestinal tract, pancreas, and kidney, among other primary sites [11, 12].
Increasingly, adrenal metastases are found by accident during cancer diagnosis, staging, and follow-up [13]. A fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) scan is useful for detecting oligometastatic disease to the AGM and excludes extra-adrenal metastatic disease if it is not evident on CT or magnetic resonance imaging. Although FDG PET/CT is not a specific marker for cancer cells, it is highly sensitive and specific in distinguishing benign from malignant tissue [14, 15].
The aim of this multicenter study was to evaluate the safety and efficacy of SBRT in patients only with oligometastatic adrenal disease or with disease progression mainly in the adrenal glands.
Materials and methods
Patient selection
In this Bulgarian multicenter retrospective study, we analyzed 75 metastatic adrenal lesions in 64 patients with oligometastatic disease only in the AGM, treated between 2015 and 2020 with the aim of achieving durable local control. We examined institutional databases for demographic, pathologic, radiologic, and treatment-related data. The hospital’s scientific research ethics committee approved the procedure.
The following criteria determined eligibility:
Pathologically confirmed diagnosis of the primary tumor;
FDG PET/CT accumulation with a maximum standardized uptake value (SUVmax) of 3.1 [15] in the AGM or only progression of the disease in the AGM is detected;
The maximum diameter of the OM was less than 5 cm;
Inoperable patients;
No history of radiation therapy for the target lesion;
Over 18 years of age;
Eastern Cooperative Oncology Group (ECOG) score of 0 or 1.
Patients were eliminated from the analysis if they received palliative treatment or dosages, or if they had further metastases.
Planning and treatment features
Planning CT was performed for each patient with a slice thickness of 1.5 mm in the supine position, and images from the planning CT and PET/CT were fused for contouring. Motion management was used in all patients with addition of an internal margin for the internal target volume (ITV). Internal target volume was defined as the contrast-enhanced lesion formed by fusing images where the dimensions of the lesion differed most, and the planning target volume (PTV) was defined as the ITV plus a margin of 1 mm. Organs at risk (OAR) were also contoured at each slice. Treatment plans were optimized according to the requirement that at least 99% of the PTV received 95% of the prescribed dose. The organs at risk limits recommended by the AAPM Task Group 101 [16] and Timmerman [17] were used. For large lesions and lesions in direct contact with adjacent critical structures, a more prolonged schedule and lower fraction dose were preferred to meet the OAR dose limits.
Total dose and fractionation schedules were determined by the treating physician based on the tolerance of neighboring normal tissues. As adrenal SBRT with larger biologically effective doses (BED) was associated with superior local control (LC), maximum tolerated doses were typically applied [18]. Before each treatment fraction, volumetric imaging guidance was used for patient positioning using kV cone beam CT.
To relate irradiation doses with clinical outcomes, the BED was calculated: a/ratio of 10 Gy was assumed for adrenal metastases. The biologically effective dose was determined using the linear-quadratic model [19]:
BED (Gy) = fractional dose x number of fractions (1+ fractional dose/α/β)
Statistical design and analysis
Data were managed and analyzed using IBM SPSS Statistics software ver. 23. The demographic characteristics were expressed as frequencies and percentages for categorical variables and as medians and means with standard deviations for quantitative variables.
The Mann-Whitney U test, χ2 test, and Kendall Tau correlation were used to compare and evaluate the correlations between response to SBRT and clinicopathological characteristics of the patients, such as age, gender, SUVmax, ITV, PTV primary tumor location and BED10. For interpretation of correlation test results, rho values were interpreted as follows: < 0.39 – weak; 0.40–0.59 – moderate; 0.60–0.79 – strong; and ≥ 0.80 – very strong.
The diagnostic accuracy of biomarkers was determined by obtaining the largest possible area under the curve (AUC) in receiver operating characteristic curve analysis. Area under the curve values: ≥ 0.9 were considered excellent, ≥ 0.80 – good, ≥ 0.7 – fair and < 0.70 – poor.
Odds ratios (ORs) with confidence intervals (CI) for categorical outcomes were calculated using a binary logistic regression model. All p-values less than 0.05 were considered statistically significant.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Approval by the local ethics committee was obtained.
Results
Baseline and treatment characteristics
In this Bulgarian multicenter retrospective study, we included 64 patients, 34 men (53.1%) and 30 women (46.9%), who were treated with SBRT for adrenal gland metastasis. The mean age of patients was 65.5 ±8.9 years at the time of diagnosis and almost all patients exhibited an ECOG performance status of 0 (90%) and another ECOG performance status of 1 (10%). We performed an analysis of 75 metastatic adrenal lesions, where 36 (48%) were non-small cell lung cancer, 9 (12%) small cell lung cancer, 10 (13.4%) rectal cancer, 8 (10.6%) renal cancer and 12 (16%) other (such as breast cancer and melanoma) oligometastatic disease. Patients were divided into three groups according to their FDG PET/CT SUVmax of adrenal metastases – with low (up to 33rd percentile, range: 3.9–7.20), intermediate (between 33rd and 66th percentile, range: 7.21–9.31) and high SUVmax (over 66th percentile, range: 9.31–25.8).
The median volume of ITV was 18.3 cm3 (range: 2.3– 148.5 cm3) and the PTV was 37.3 cm3 (range: 5.8–224.5 cm3).
The median dose regimens were 35 Gy delivered in 1–5 fractions, with the median dose of PTV being 35 Gy (range: 16–48 Gy) and the BED10, (α/β = 10) being 59.5 Gy (range: 35.7–124.8 Gy). Overall, the objective response rate (ORR) – the proportion of patients who had a partial or complete response (CR) to the treatment – was 40%.
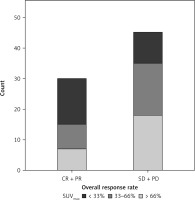
All clinicopathological characteristics such as gender, age, BED10, ITV, PTV, SUVmax etc. were analyzed as predictive factors for ORR. We detected a significant relationship between ORR and PTV (p = 0.024), SUVmax (p = 0.042) and localization of the primary tumor (p = 0.008) (Table 1). The levels of SUVmax and ORR were moderately significantly related (Kendall Tau-c = 0.29; p = 0.017) (Fig. 1).
Table 1
Relationship between baseline clinicopathological characteristics of patients and response to stereotactic body radiation therapy
SUVmax as a non-invasive biomarker for the discrimination between patients with or without response
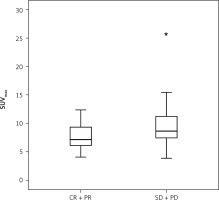
The Mann-Whitney U test showed that patients with progressive disease (PD) and stable disease (SD) had significantly higher levels of SUVmax than patients with CR and a partial response (PR) (7.6 ±2.4 vs. 9.7 ±3.8; p = 0.015) (Fig. 2).
Fig. 2
Bar graph depicting the number of patients with complete response and partial response vs. patients with progressive disease and stable disease. The Mann-Whitney U test was used to detect significant differences in the levels of maximum standardized uptake value between the groups (p = 0.015)
CR – complete response, PD – progressive disease, PR – partial response, SD – stable disease, SUVmax – maximum standardized uptake value
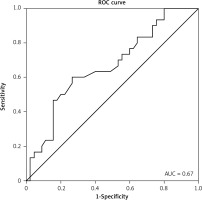
Receiver operating curve analysis was performed to explore the potential predictive role of SUVmax as a non-invasive biomarker for discrimination between patients with or without a response. At the optimal cut-off values for SUVmax, the biomarker could significantly and acceptably distinguish between patients with or without a response (AUC = 0.67, 95% CI: 0.54–0.79; p = 0.015), with a sensitivity of 63.5% and a specificity of 60.1% (Fig. 3). There was no significant association between cancer type and SUVmax.
Fig. 3
At the optimal cut-off values for maximum standardized uptake value, the biomarker could significantly and acceptably distinguish between patients with or without response (AUC = 0.67, 95% CI: 0.54– 0.79; p = 0.015), with a sensitivity of 63.5% and a specificity of 60.1%
ROC – receiver operating curve
Predictors of response rate to stereotactic body radiotherapy
In the univariate binary logistic regression model, we found that SUVmax as a continuous variable was associated with a poor response to SBRT (OR, 1.28; 95% CI: 1.056–1.568; p = 0.012). When SUVmax increased by one unit, there was a 28% greater chance of a poor response, although this significant loss was in the multivariate analysis. A small PTV was a significant predictor of a good response (OR, 0.33; 95% CI: 0.127–0.875; p = 0.026) (Table 2).
Table 2
Univariate logistic regression analysis for predicting response to stereotactic body radiation therapy
Discussion
A growing number of tiny retrospective series on the outcomes of SBRT for adrenal metastases have been published in recent years [20–27]. These studies have small sample sizes, which hinders the estimation of treatment efficacy and the determination of optimal dosimetry details.
In this study, the relationship between SBRT responsiveness and SUVmax level in patients with oligometastatic adrenal disease was evaluated, revealing prospective survival benefits and excellent LC. When SUVmax increased by one unit, the probability of an inadequate response to SBRT increased by 28%. SUVmax levels were considerably higher in patients with PD and SD than in patients with CR and PR. In addition, we discovered a significant correlation between ORR and PTV, SUVmax, and primary tumor location.
Metastases to the AGM are common and challenging to distinguish from benign adenomas. Adrenal metastases are typically asymptomatic, and up to fifty percent of patients with confirmed cancer may have benign disease; therefore, noninvasive characterization is necessary to prevent unnecessary biopsies [14]. Although FDG PET/CT is not a specific marker for cancer cells, it is highly sensitive and specific in differentiating benign from malignant adrenal tumors in patients with known primary malignancies.
Metser et al. [15] found that FDG PET/CT had a sensitivity and specificity of 98.5% and 92%, respectively, for distinguishing lipid-poor adenomas from malignant lesions, and 98.5% and 93% for distinguishing all adenomas from malignant lesions. Boland et al. [19] observed that PET/CT had greater sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for the detection of benign lesions and for the detection of malignancy. Metser et al. also discovered that when SUVmax = 3.1 was used as a cutoff, FDG PET/CT exhibited a sensitivity and specificity of 98.5% and 92%, respectively, for differentiating malignant from benign tumors [15].
In a meta-analysis of ten investigations, nine of which were retrospective, the 1- and 2-year LC rates for patients with a median BED10 60 Gy were greater than 70% [28]. Similarly, in a recent meta-analysis involving > 1,000 patients from 39 studies, SBRT was associated with an 82% 1-year LC rate with an outstanding safety profile, and a higher SBRT dose was strongly associated with an improvement in LC [9]. In the most recent and largest retrospective series of adrenal SBRT published to date, Buergy et al. [29] reported the outcomes of 326 patients with adrenal metastasis, where the 1-year LRFS rate for 260 patients treated with SBRT was 80%. The lung was the predominant primary tumor site, and the median BED10 was greater than 50 Gy in all of these studies. In only a handful of studies has the efficacy of SBRT for AGM been investigated; however, in this analysis, we present the correlation between FDG PET/CT SUVmax and response to SBRT.
Franzese et al. found that tumor origin was an independent survival factor [30]. Metastases from colorectal cancer had higher LC rates than metastatic lesions from other malignancies.
Despite quicker amelioration of symptoms in patients with AGM from lung cancer, their prognosis was like those with AGM from other malignancies. This may be a result of the disparity in the differentiation and radiosensitivity of AGM tumors. Therefore, it may be suggested that tumor origin is unrelated to the efficacy and prognosis of SBRT, making SBRT a suitable treatment method for metastases regardless of their origins. We found no statistically significant associations between cancer type, SUVmax, and SBRT response. However, the results should be validated by further research.
The tumor ORR is the evaluation of the tumor burden in patients with solid tumors following a certain treatment. The metabolic response evaluations using FDG PET/CT may reflect the viability of cancer cells or functional changes occurring after anticancer treatments and may indicate the efficacy of a treatment before anatomic shrinking [31].
We recorded an ORR rate of 40%, which is comparable to previous research [24, 32–36]. Complete remission and PR were observed in 30 (40%) lesions according to RECIST criteria, whereas PD and SD were observed in 45 (60%) individuals.
Similar to the research by Toesca et al., one of the prognostic parameters for OS in our study is tumor diameter [27]. Chawla et al. analyzed adrenal SBRT in 30 patients and reported an ORR of 55%; [21] another study involving 33 patients revealed an ORR of 50% [37]. Both Scouarnec et al. and Toesca et al. [27, 38] reported an ORR of 80%, which was preferable. However, their irradiated tumors had a median volume of 13.1 and 19 cm3, whereas in our study, the median tumor volume was significantly higher at 18.3 cm3 for the ITV and 37.3 cm3 for the PTV. Konig et al. [39] examined 28 adrenal metastases treated with SBRT, where the ORR was 86% and the median tumor volumes for the ITV and PTV were 42 cm3 and 96 cm3, respectively.
Previous research [35, 40] has demonstrated that the LC is enhanced when the BED10 is > 100 Gy for primary lung cancer and > 70 Gy for primary pancreatic cancer. Considering these findings, several authors studied the effect of a higher BED10 on adrenal metastases and found that higher doses were associated with a superior LC [21, 41]. Chance et al. [33] reported no local failures following adrenal SBRT with a BED10 > 100 Gy in which 84% of cases had lung cancer as the primary tumor. Moreover, in the SABR-COMET study, which resulted in enhanced survival for oligometastatic disease, adrenal metastases were treated with a BED10 dose greater than 100 Gy [42]. In the study by Scouarnec et al. [38], in which more than 80% of patients were irradiated with BED10 > 100 Gy, it was reported that the LC rates were higher than in other comparable series in the literature. Several studies have documented improved outcomes with a BED10 threshold between 72 Gy and 90 Gy [28, 43]. In our study, patients with a BED10 greater than 59.5 Gy and a prescribed dose greater than 27 Gy in 3 fractions or 35 Gy in 5 fractions had a superior ORR.
Conclusions
Our study has several limitations, including its retrospective nature, limited sample size, and inclusion of patients with diverse tumor forms. Despite these limitations, our data demonstrate that not only may FDG PET/CT be used to plan SBRT, but also SUVmax may be employed as a noninvasive biomarker to distinguish between responder and nonresponses patients. This may aid doctors in predicting responsiveness to SBRT and in the hunt for radiosensitizers to enhance ORR in patients with higher SUVmax values.