Introduction
According to the Centers for Disease Control and Prevention (CDC), trauma is the leading cause of death in individuals during the first four decades of life and the third leading cause of death across all age groups [1]. Following traumatic brain injury and spinal cord injury, thoracic trauma is the third most common site of trauma [2]. Thoracic injuries can involve the chest wall (rib and sternum fractures) or mediastinal organs, including the major vessels, heart, lungs, esophagus, trachea, and diaphragm.
Thoracic trauma accounts for 25% of all trauma-related deaths in the United States, and its presence in polytrauma is associated with a 50% increase in mortality rates [3]. Mortality is particularly high when the heart, aorta, esophagus, or tracheobronchial tree is involved. Thoracic trauma contributes to immediate fatalities due to damage to the heart or aorta, as well as late in-hospital deaths caused by respiratory complications and infections. Interestingly, even though 50% of thoracic injuries are minor, one in 3 cases requires hospital admission [4].
Lung parenchymal injuries are a common outcome of major chest trauma, with their severity closely related to the mechanism of injury [5]. The predominant cause of lung injury is blunt chest trauma, accounting for 80–92% of cases [6, 7]. Although less common, penetrating trauma can be life-threatening. Prompt recognition and effective management are crucial to prevent both short- and long-term respiratory and systemic complications, ensuring optimal patient outcomes.
This review endeavors to provide a comprehensive understanding of lung parenchymal trauma, focusing on the biomechanics, mechanisms, and classification of injuries, while emphasizing the importance of accurate diagnosis and appropriate management strategies.
Material and methods
This study systematically reviews the biomechanical properties of lung parenchymal tissue in response to thoracic trauma. A comprehensive literature search was conducted using databases such as PubMed, Scopus, and Web of Science, focusing on articles published in the past two decades. Studies were selected based on relevance to lung injuries classified as blunt, penetrating, and blast trauma. Emphasis was placed on research articles, clinical trials, and reviews that discussed lung contusions, lacerations, herniations, and vascular injuries.
Imaging data, including computed tomography (CT) scans, X-rays, and ultrasounds, were analyzed to illustrate and diagnose different types of lung parenchymal trauma. The review also incorporated clinical guidelines and management strategies for diagnosing and treating lung trauma, with a focus on surgical interventions and rehabilitation outcomes.
Histological analysis of lung tissue involved examining published studies on extracellular matrix (ECM) responses to trauma. Biomechanical models and tissue damage pathways were reviewed to understand how mechanical forces such as acceleration, deceleration, and pressure waves impact lung structure and function. The findings are organized into categories based on injury mechanism and severity.
Discussion
Mechanics of lung trauma
Trauma biomechanics examines how mechanical forces affect various tissues with differing properties and lead to cellular changes. Understanding the mechanism of injury and basic kinetic principles allows for more accurate predictions of outcomes and facilitates well-informed diagnoses, ultimately improving patient care.
Newton’s laws of motion provide the foundation for understanding interaction and energy conversion within a closed system. The first law states that a change in an object’s motion requires the application of a force. The second law states that the net force acting on an object is equal to its mass multiplied by its acceleration (F = ma). The third law, most pertinent to trauma, asserts that the total energy in a closed system remains constant. When energy transfer exceeds a tissue’s intrinsic endurance, tissue trauma occurs. Key factors influencing trauma include mass, velocity (K = 1/2mv2), density, and the surface area of the involved objects.
Lung susceptibility to trauma is influenced by several factors, including its size within the mediastinum, the delicate branching structure of the bronchi into pulmonary alveoli, and the microanatomy of lung tissue.
Histologically, pulmonary alveoli, the major sites of gas exchange, which are responsible for the spongy nature of the lung, consist of a monolayer of type I (95%) and type II pneumonocytes, a thin layer of ECM, capillary endothelium and other cells including alveolar macrophages and fibroblasts. The pulmonary extracellular matrix (ECM) consists of numerous proteins (e.g. collagens, proteoglycans, glycoproteins), called the “matrisome” [8]. This network is primarily composed of elastin and collagen, which together play complementary roles in determining the biomechanical properties of the lung, particularly elasticity and tensile strength [9]. The ECM is dynamically linked to cellular behavior, modulating cell function, morphology, and differentiation [10, 11]. It also serves as a reservoir for growth factors and cytokines (e.g., FGF, TGF-β1) and facilitates signal transduction through ECM-cell adhesion, which regulates lung tissue homeostasis and behavior [11]. Post-translational modifications of the ECM proteins ensure their stability and functionality and consequently are an important molecular player of the ECM roles [12]. Furthermore, ECM degradation, primarily mediated by matrix metalloproteinases (MMPs), is essential for lung tissue repair and regeneration after acute lung injury (ALI). Particularly, MMP7 plays a critical role in the injury response in mucosal epithelia, regulating cell-cell and cell-matrix adhesions by shedding of E-cadherin and syndecan-1 from the cellular surface [13, 14].
Consequently, it becomes clear that the lung’s connective tissue is not a static structure, but the result of a dynamic balance between constant breakdown and remodeling [11]. Alterations in the micro- and macro-environment, such as traumatic changes to the lung, can disturb the tissue equilibrium and trigger dynamic remodeling via the constant production and breakdown of the macromolecules of the ECM.
Notably, gender differences in respiratory physiology also affect lung biomechanics [15]. Thoracic trauma accounts for 25% of trauma-related deaths, with 12% occurring in men, compared to 7% in women [16]. As proposed by Rizzo et al. [16], this discrepancy may be attributed to differences in sex hormone levels, particularly the protective effects of estrogen, which are associated with enhanced elastin responses in women, as demonstrated using confocal laser scanning microscopy.
Respiratory compliance, a measure of the lung and chest wall expandability, is another factor affecting lung biomechanics. Respiratory compliance consists of two components: chest wall compliance and lung compliance. Compliance is closely related to the fragility of the lung [3]. Higher lung compliance correlates with greater energy absorption in the lung parenchyma, while reduced compliance shifts energy absorption to the bony thorax. In older patients, the risk of thoracic fractures increases due to decreased chest wall compliance from ossification. Conversely, pediatric patients experience a higher incidence of significant lung injuries (up to 80%) due to increased chest wall compliance, allowing the thorax to better withstand compression [17].
Additionally, regional variability in lung architecture affects its mechanical behavior [18]. The lung parenchyma is more uniform in the periphery, where alveolar sacs predominate, compared to the central regions, where bronchial branching occurs [19].
Lung trauma mechanisms
Trauma can primarily be categorized into two groups: blunt and penetrating. Blast injury represents a more complex category, as it combines elements of both blunt and penetrating injuries, depending on the phase of blast trauma [2]. Each type of trauma follows its own injury mechanism, based on physical principles such as the conservation of energy and Newton’s Third Law. In most cases, kinetic energy is transformed into distortion energy, leading to tissue damage.
Blunt trauma
The vast majority of thoracic trauma cases involve blunt injuries, with motor vehicle collisions accounting for 70% to 80% of incidents. Other causes include falls from height, occupational accidents, roadside accidents, contact sports, extreme sports, and acts of violence [20]. In blunt trauma, the integrity of the tissue is generally maintained, but the injuries often result in contusions and lacerations. These can lead to significant morbidity and mortality, with life-threatening complications that require timely and appropriate management [21, 22]. Less than 10% of blunt trauma cases necessitate surgical intervention [23], while approximately 90% are managed effectively with chest tube insertion [24].
As previously noted, the patient’s age is a critical factor in evaluating blunt trauma, as older individuals face a higher risk of thoracic fractures due to reduced thoracic elasticity [25]. The elderly are particularly vulnerable to blunt thoracic trauma because of their decreased pulmonary function and capacity [26].
Blunt chest trauma can result from four primary mechanisms: 1) direct impact to the chest, 2) thoracic compression, 3) acceleration/deceleration injuries, and 4) blast injuries [27].
Direct impact to the chest typically causes localized injury to the chest wall (e.g., rib fractures, flail chest), which can affect the underlying intrathoracic structures. If the lung parenchyma is involved, the injuries may include lung contusions, lacerations, hematomas, pseudocysts, and hemo- or pneumothorax.
Compression injuries occur when intrathoracic organs and compressible structures, such as blood vessels, are compressed against fixed thoracic structures (spine, ribs, sternum). These injuries are commonly caused by crush incidents, occupational accidents, or falls from height.
Acceleration/deceleration injuries are typically associated with high-speed motor vehicle accidents and falls from height. In such cases, the body is abruptly halted, while the internal organs continue moving at high velocity, leading to the tearing of vessels and tissues at their points of attachment, as explained by Newton’s first law of motion [28].
Blast injuries
As the risk of terrorist bombings and warfare increases, blast injuries are expected to become more prevalent in clinical practice. Yen et al. [29] suggest that the lung is the most susceptible organ to blast-related trauma. Explosions create distinct injury patterns, with “blast lung” being the most common fatal injury among survivors. This condition can result in both blunt and penetrating trauma [30]. The blast wave generates overpressure, resulting from the energy released during the initial explosion [31].
Blast injuries are categorized into four phases: primary, secondary, tertiary, and quaternary [32]. The primary phase is caused by exposure to blast overpressure (a sudden rise in pressure) following an explosion [33]. The shock wave can travel at velocities up to 3000 m/s [34]. Maximum energy transfer occurs at points where tissue density changes, making gas-filled structures – such as the larynx, lungs, middle ear, gastrointestinal tract, and eyes – particularly vulnerable to blast injury [35]. As tissue density changes, the velocity of the pressure wave is also altered, either accelerating when moving from high- to low-density areas or decelerating when moving in the opposite direction. These velocity changes increase tissue damage due to the absorption of energy in surrounding tissues. In the chest wall, the blast wave accelerates when entering and leaving the lungs, which are air-filled and have lower density than the surrounding structures (e.g., bony thorax, muscles, fat) [36].
Blast lung, as defined by Mackenzie et al., is “acute lung injury within 12 hours of blast exposure, not due to penetrating or blunt injury” [37]. It is characterized by alveolar rupture, parenchymal hemorrhage, and edema, as well as lung contusions, lacerations, pneumothorax, pneumatoceles, and bronchopleural fistulas. These injuries compromise lung ventilation and contribute to respiratory failure in blast lung [38, 39]. This condition can lead to sudden respiratory deterioration and tissue hypoxia. The secondary phase of blast trauma is caused by bomb fragments, leading to both penetrating and blunt lung injuries. The tertiary phase involves blunt injuries caused by the body being thrown into objects by the blast wind [40]. Finally, quaternary phase injuries include burns, chemical exposure, and inhalational injuries [28].
Penetrating trauma
Penetrating trauma is less common than blunt trauma but typically more lethal. It occurs when a sharp object applies force to a specific area, damaging underlying tissues and disrupting their integrity, resulting in an open wound [41]. Clinical presentations can range from asymptomatic with minor superficial wounds to severe cases of hemodynamic instability [42].
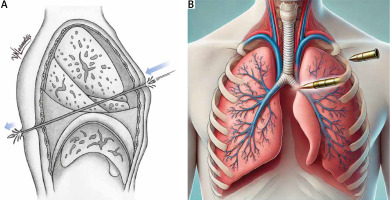
Penetrating wounds can be ballistic (gunshot wounds, fragments from explosives) or non-ballistic (knife, scissors, nails, or other sharp objects). Impalement injuries, which involve significant force, occur when the patient’s body weight falls onto an object (Figure 1).
Figure 1
Diagram illustrating a penetrating thoracic injury inflicted by a knife, demonstrating the path of the blade through the thoracic cavity
The severity of penetrating trauma is determined by the kinetic energy of the penetrating object and the type of weapon used [43]. Penetrating chest wounds can be further categorized as high-energy injuries (velocities > 600 m/s), intermediate-energy injuries, or low-energy injuries (velocities < 334 m/s) [44, 45].
The wound’s location within the mediastinum is of particular clinical significance. Life-threatening complications requiring immediate treatment include airway obstruction or rupture, tension pneumothorax, massive hemothorax, and pericardial tamponade [46]. Vascular injuries, most frequently involving the ascending aorta, aortic arch, and proximal great vessels, present significant clinical challenges and require prompt management [47].
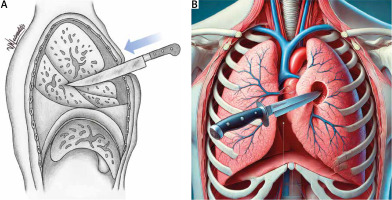
Gunshot wounds can be either penetrating (where the bullet does not exit the body) or perforating (where the bullet passes through the body). These injuries cause extensive tissue destruction along the projectile’s path (Figure 2). When a high amount of energy is transferred to the lung parenchyma, cavities form. The ‘permanent pulmonary cavity’ or ‘tunnel of attrition’ [48] represents the projectile’s path through devitalized tissue, manifesting as a pulmonary laceration. The ‘temporary cavity’, on the other hand, refers to tissue contusion caused by alveolar hemorrhage and edema in the surrounding tissues due to energy absorption rather than the direct trajectory of the projectile [45, 49].
Classification of lung parenchymal injuries
Lung parenchymal injuries include lung contusions, lung lacerations (which may lead to the formation of hematomas and pneumatoceles), lung herniation, and vascular injuries of the lung.
The Organ Injury Scaling Committee of the American Association for the Surgery of Trauma has developed the Lung Injury Score (LIS) which classifies lung injuries into six grades (I–VI) [50] (Table I).
Table I
Lung Injury Score (LIS)
Lung contusion
First described in 1761 by the Italian anatomist Morgagni as lung injury with an ‘intact thorax’ [51] and defined by the French military surgeon Guillaume Dupuytren in the 19th century [52], lung contusion is an important clinical entity constituting up to 75% of blunt chest trauma cases.
Lung contusion refers to direct or indirect traumatic damage to the lung parenchyma, resulting in edema and alveolar hemorrhage without structural damage to the lung architecture, though with significant impairment of lung function [53]. This injury occurs due to fluid leakage from disrupted capillaries in the alveolar walls and septa, allowing blood to flow into the alveolar spaces and interstitium [54].
Pulmonary contusions are typically due to blunt trauma, but blast trauma could also be the cause. High-velocity acceleration/deceleration such as motor vehicle collisions constitute the majority of cases.
In more than 75% of cases, lung contusions are not isolated injuries but are associated with other chest injuries, including rib fractures, flail chest, and hemo- or pneumothorax, particularly when there is tearing or penetration of the visceral pleura [55]. In 1975, Trinkle et al. [56] demonstrated that the respiratory defects observed in patients with flail chest were primarily due to underlying lung contusions rather than chest wall instability.
Even when a lung contusion is unilateral, the contralateral lung parenchyma can also be affected due to increased inflammation, which can rapidly progress to acute respiratory distress syndrome (ARDS). This demonstrates that lung contusion is not a localized pathology, but rather one that can have both pulmonary and systemic effects when a significant portion of the lung is involved. Lung trauma triggers a robust innate inflammatory response, characterized by leukocyte recruitment, activation of tissue macrophages, and the production of inflammatory cytokines by immune and pulmonary cells. Neutrophil activation via Toll-like receptors (TLRs) such as TLR2 and TLR4, along with fibroblast activation and proliferation, further damages the lung epithelium and disrupts gas exchange [57–59].
In most cases, following trauma, the lung may be injured at the site of impact (coup injury). In rarer cases, such as when striking a stationary object or in blast injuries, the lungs may violently collide against the thoracic cage on the opposite side of the impact, resulting in a contrecoup injury [60].
The flexibility of the chest wall determines whether energy from trauma is absorbed by the bones or transferred to the lung parenchyma. In younger patients, a more flexible chest wall can result in severe, diffuse lung contusions without rib fractures [61].
Clemedson and Pettersson [62] described three primary mechanisms that explain how a shock wave passing through the lung can cause a contusion: the inertial effect, the spalling effect, and the implosion effect [63].
The pathophysiology of lung contusion, often referred to as “traumatic wet lung syndrome”, involves increased alveolar permeability, leading to fluid leakage and edema, which reduces the effective respiratory surface area and causes hypoxia. Other effects include increased pulmonary vascular resistance, altered ventilation and perfusion, increased intrapulmonary shunt, and reduced lung compliance [64].
Clinically, lung contusions typically present with symptoms of hypoxia due to atelectasis, hypercarbia, and increased work of breathing. Additional symptoms may include dyspnea, tachycardia, tachypnea, wheezing, hemoptysis, difficulty breathing, and diminished breath sounds in the affected lung area [65].
The clinical presentation of lung contusion can be insidious, so it is important to reassess vital signs and ensure airway protection. Symptoms may be absent immediately after the injury but can develop rapidly or within 24–48 hours as the injury progresses from an edematous phase to alveolar collapse and lung dysfunction [66].
Chest X-ray is typically the initial imaging method used for diagnosis and triage. However, radiographic findings may not be evident within the first 6 hours following trauma, becoming more pronounced at 24 hours when edema and hemorrhage are significant. Therefore, repeat imaging is recommended [67, 68]. The expansion of lung contusions within the first 24 hours is considered a poor prognostic indicator. Common findings include focal or diffuse homogeneous opacifications across multiple lung segments or lobes (“patchy consolidations”), with the opacities not adhering to normal anatomical boundaries [66].
Lung ultrasound (LUS) is a valuable tool for rapid bedside imaging in cases of hemodynamic instability, inconclusive X-ray results, or when computed tomography (CT) is not feasible. In emergency settings, LUS has been shown to be 10% more accurate than X-ray and clinical examination combined in identifying lung contusions. It allows clinicians to observe lung pattern variations as the injury progresses [69].
CT remains the gold standard for assessing parenchymal pathologies and can detect lung contusions as early as the initial imaging [67, 68]. CT is 38–81% more accurate than X-ray in diagnosing lung contusions. On CT, contusions appear as crescentic, focal, non-segmental areas of opacification without cavitation, often accompanied by a ground-glass appearance and sparing of the subpleural region. CT also aids in evaluating the severity of lung contusions [70]:
Mild lung contusion: < 20% of lung volume affected.
Moderate lung contusion: 20–50% of lung volume affected.
Severe lung contusion: > 50% of lung volume affected.
Mortality associated with lung contusion ranges from 14% to 40% [71]. In most cases, lung contusions resolve without complications within 3 to 7 days [64]. In trauma patients, lung contusions are independently associated with an increased risk of ARDS [72], while the risk of pneumonia is elevated due to the lung’s impaired ability to clear secretions and bacteria [66].
Moderate to severe pulmonary contusions are associated with a higher risk of ARDS [73], prolonged mechanical ventilation, extended ICU stays, and longer overall hospitalizations [74]. Scoring systems such as the Abbreviated Injury Scale (AIS) and BPC18 are effective tools for evaluating contusions and their association with worse outcomes [74]. The Murray Score, which assesses four criteria – degree of hypoxemia, lung compliance, radiographic findings, and positive end-expiratory pressure (PEEP) – is used to score the risk of ARDS development [75].
The management of pulmonary contusion is primarily supportive, including oxygen therapy and careful pain management, as thoracic pain can contribute to hypoventilation [63]. Diuretics are often used to manage edema [65]. Fluid administration is controversial, as both hypovolemia and fluid overload can be detrimental; thus, careful fluid management is essential [64]. The routine use of prophylactic antibiotics and steroids is not supported by clinical evidence. Respiratory exercises and physiotherapy are important to improve lung expansion and prevent complications such as atelectasis and pneumonia [63].
Lung lacerations
Pulmonary laceration is defined as a tear in the lung parenchyma, resulting in the formation of a well-circumscribed opacity or cavity that may become filled with air (pneumatocele or traumatic pseudocyst), blood (pulmonary hematoma), or, more frequently, a combination of both [76]. Unlike pulmonary contusions, lacerations involve a disruption of the lung’s architectural integrity.
Pulmonary lacerations are primarily associated with penetrating trauma [77], but they can also occur as a serious consequence of severe blunt chest trauma, with a reported prevalence of 4.0% to 4.4% in all cases of blunt chest trauma. This includes pleural or lung perforation due to rib fractures or inertial deceleration [78, 79]. These injuries are most commonly observed in patients under 30 years old, owing to the greater compliance of the thoracic cage in younger individuals [80].
In 1977, Hankins et al. [79] described two mechanisms by which blunt trauma can cause laceration: a direct one (direct tearing of the lung by the sharp edges of broken ribs) and an indirect one (compression and decompression).
Clinical symptoms can range from minimal to severe, including cough, chest pain, hemoptysis or blood-stained sputum, dyspnea, tachypnea, and hypoxemia [81]. Since clinical features are often non-specific and may not be immediately apparent, the diagnosis requires a high degree of suspicion. Missed diagnoses can pose a challenge for clinicians [81, 82]. Complications such as pneumothorax, hemothorax, or hemopneumothorax may occur if rib fractures disrupt the pleura, establishing communication between the lung parenchyma and the pleural space.
On initial chest radiographs, approximately 50% of pulmonary lacerations may go undetected as they are often obscured by surrounding pulmonary contusions. These lacerations typically become visible 48–72 hours later as the contusions resolve [83]. The resulting cavity may be filled with blood or air, forming traumatic pulmonary cavitary lesions. If both air and blood are present, an air-fluid level can be seen.
CT has greater sensitivity and specificity in identifying lacerations [84]. Pulmonary lacerations often appear as solitary lesions but can sometimes present as multiple small lung cysts, giving the lungs a “Swiss cheese” appearance [83, 84]. Unlike the linear appearance of lacerations in other organs, pulmonary lacerations appear as round or oval lesions on CT, due to the elasticity of lung tissue, which retracts from the site of injury.
Wanger et al. [85] divided pulmonary lacerations into four types based on the causal mechanism and the CT findings (Table II).
Table II
Wanger’s lung laceration classification based on CT
Type I lacerations are the most common, followed by Type III, with both types predominantly seen in younger patients due to higher respiratory compliance [86]. Type IV injuries are the rarest and may not always present obvious signs of lung injury, even on CT scans [87].
Pulmonary lacerations generally heal after chest tube insertion for the removal of air or blood, without significant long-term effects [88]. Specific therapy is typically not required unless complications arise, and only a small proportion of patients require surgical intervention. Indications for surgery include intrathoracic hemorrhage with more than 1,000 ml of immediate blood drainage during initial tube thoracostomy or 200 ml/h for four consecutive hours, massive air leakage with failure of lung re-expansion, and ventilation failure due to airway bleeding [89]. Surgical procedures may involve suturing or wedge resection, with lobectomy or pneumonectomy being rare and reserved for severe cases [83]. Video-assisted thoracoscopic surgery (VATS) is commonly used in the management of acute thoracic trauma, except in cases of hemodynamic instability, where thoracotomy is required [87, 90]. For patients with pulmonary lacerations requiring general anesthesia, there is an increased risk of pneumothorax formation or conversion to tension pneumothorax during mechanical ventilation. In such cases, thoracic surgeons must be prepared to manage these complications effectively.
Complications from pulmonary lacerations are uncommon but can include abscess formation, bronchopleural fistula, and enlargement of the laceration. Abscess formation typically presents with the classic signs of infection. A bronchopleural fistula occurs when there is communication between the laceration, a bronchiole, and the pleural space, causing air leakage despite the presence of a chest tube. Enlargement of the pulmonary laceration can further compress the lung and worsen respiratory function [88].
Traumatic pulmonary cavitary lesions
Intrapulmonary hematoma
Intrapulmonary hematomas are formed from a laceration that becomes filled with blood. They are called “coin” lesions because of their round and oval appearance. They might result from direct blunt trauma or blast injury. Initially, round hematomas may be completely obscured by surrounding lung contusions for several days to weeks [91, 92]. They usually resolve in approximately 2 to 4 weeks, but they may become secondarily infected and present as an abscess [93]. In rare cases, intrapulmonary hematomas can persist for extended periods as slowly expanding, space-occupying masses, necessitating the exclusion of malignant or metastatic disease [94, 95]. Complications such as abscess formation, uncontrolled hemorrhage, or massive hemoptysis may occur, potentially requiring surgical intervention [96].
Post-traumatic lung pseudocyst
First described by Fallon in 1940 [97], the post-traumatic lung pseudocyst, also known as a pneumatocele, is an acute disruption of the pulmonary parenchyma that lacks epithelial lining, forming one or more intrapulmonary air-filled spaces [98]. This uncommon cavitary lesion is observed less frequently than intrapulmonary hematomas, typically forming after blunt trauma and rarely following penetrating injuries [99]. The exact mechanism of formation is unclear, but it is thought that air enters the lacerated parenchyma through a check-valve mechanism and becomes trapped in the pleural space [100]. Unlike hematomas, pseudocysts are usually identified by the appearance of air within 48 hours after injury [101]. Young adults and children are most commonly affected [99], as the flexibility of the bony thorax in these age groups permits the compression and disruption of the lung, leading to pseudocyst formation [98]. These lesions typically resolve spontaneously within 2 weeks to 5 months. Pulmonary hematoma and traumatic pulmonary pseudocysts are closely related; if a pulmonary hematoma establishes communication with an airway, it can convert into a pseudocyst, with hemoptysis as a possible presenting symptom [102].
Traumatic lung herniation
Traumatic lung herniation is a rare entity, with only 300 cases reported [103]. Intercostal lung hernia was first defined as a protrusion of the lung through an abnormal opening in the chest wall by Roland in 1499 [104]. There are two factors predisposing to hernia creation; increased intrathoracic pressure and anatomical weakness of the chest wall. Defects in the thoracic wall such as multiple rib fractures and flail chest or sternoclavicular or costo-chondral dislocations are examples of factors responsible for chest wall weakness [76] (Figure 3).
Figure 3
Imaging presentation of a substantial herniation of the left upper lung lobe through the third intercostal space (indicated by arrow). A – Computed tomography (CT) scan, B – chest X-ray demonstrating the extent of the herniation

The Morel-Lavallée classification system is used to categorize lung hernias based on their location (cervical/supraclavicular, thoracic/intercostal, diaphragmatic, mediastinal) and etiology (congenital or acquired) [105]. Acquired intercostal lung hernias are further classified as traumatic, spontaneous, iatrogenic, or pathological [106, 107]. Approximately 20% of lung hernias are congenital, while 80% are acquired, with most cases being traumatic in origin [108]. Hernias most commonly occur in the anterior thoracic wall, where the intercostal muscle layer is thinner and lacks the support of the posterior thoracic muscles [106]. The parasternal area, composed of cartilaginous rib segments, is the most common site for intercostal hernias.
Traumatic lung herniation is typically a consequence of blunt trauma, even from low-energy forces [109]. Motor vehicle accidents are the most frequent cause, though cases related to falls and seatbelt injuries have also been reported [110]. Herniation can manifest immediately after the injury or as late as 50 years later, as described by Hamidian Jahromi et al. [111] and Yadav et al. [112].
Hernias are more common in males, and patients with chronic pulmonary diseases such as COPD and emphysema are at increased risk due to chronically elevated intrathoracic pressures and chronic coughing [105, 113]. Other risk factors include obesity, heavy lifting, and activities that raise intrathoracic pressure, such as playing wind instruments. Tissue weakness associated with conditions such as diabetes, malnutrition, and steroid use also contributes to hernia formation [108].
Clinical symptoms can range from asymptomatic to chronic thoracic pain, dyspnea, shortness of breath, and acute thoracic pain when inspiring, sneezing, or coughing.
Diagnosis is typically achieved through a combination of physical examination, imaging studies, and lung function tests [105]. On physical examination, a soft mass that increases in size with elevated intrathoracic pressure, such as during a Valsalva maneuver, may be observed on the chest wall [104]. Chest X-ray may reveal lung herniation as a well-circumscribed area of subcutaneous air [114] and is characterized by the presence of lung tissue outside the rib cage, also known as the “lung beyond rib sign” [115]. CT scans are more accurate in determining the exact size and location of the hernia and in ruling out other associated injuries [115, 116]. Additionally, eFAST (extended Focused Assessment with Sonography for Trauma) can contribute to the rapid diagnosis and identification of complications such as pneumothorax [116].
While traumatic lung hernias are rarely life-threatening, they can be associated with complications such as parenchymal ischemia, pneumothorax, acute respiratory distress syndrome (ARDS), pneumonia, systemic inflammatory response syndrome (SIRS), atelectasis, and chronic pain syndrome, as proposed by Leivaditis et al. [105].
Intercostal lung hernias have a very low possibility of spontaneous resolution, and treatment options include observation and surgery [116]. Presence of symptoms, location and hernia size are important factors in treatment decision-making. Conservative treatment is preferable for small asymptomatic hernias with no complications. Indications for surgical repair are incarceration of lung tissue, chronic pain, increasing size, recurrent respiratory infections and respiratory failure [104, 117]. Early surgical repair is associated with better results and short- and long-term prognosis [114, 115]. Thoracotomy is more widely used than the thoracoscopic approach [118]. Surgical options include primary repair, using mesh, muscle, or a periosteal flap to bridge the defect, while VATS can help to confirm the viability of the lung tissue [115, 117].
Pulmonary vascular injuries
Injury to the main pulmonary arteries (PA) and pulmonary veins (PV) is most commonly caused by penetrating trauma, such as stab wounds [119]. However, sudden deceleration from blunt trauma can also cause these lesions, such as tearing at the junction between the pulmonary veins and the left atrium [120]. Available data are limited because major injuries to the principal branches of the pulmonary arteries or veins are associated with a high mortality rate, exceeding 75% [86]. Many deaths occur either at the scene of the accident or shortly afterward, primarily due to exsanguinating hemorrhage [119].
Complex lesions involving both vascular and pulmonary structures, the heart, or other mediastinal vessels can be rapidly fatal, often resulting in hemodynamic instability and requiring pneumonectomy to control bleeding [120–122]. However, isolated vascular injuries in hemodynamically stable patients have a better prognosis and can often be managed with surgical vascular repair [119, 122].
The pulmonary arterial system is characterized by relatively low pressure. Clinically, PA injuries most commonly present with hemodynamic instability, massive hemothorax, or hemoptysis [121], and often reveal a pseudoaneurysm on computed tomography angiography (CTA) [120]. Even small branch injuries, such as transection or rupture, can cause fatal exsanguination within seconds but may also be controlled with the application of gentle compression [123]. Dyspnea may accompany hemothorax. In cases of intrapericardial PA injury, patients often present with cardiac tamponade [124]. Pulmonary vein rupture can be particularly insidious, as patients often remain hemodynamically stable [125]. This is because the low-pressure hemothorax formed in the pleural cavity acts as a “giant left atrium”, as proposed by Varghese et al. [126]. In these cases, the insertion of a chest tube to drain the hemothorax can lead to rapid volume depletion and worsen the patient’s condition, whereas retaining the hemothorax may provide hemodynamic stability. In contrast to the venous system, the higher-pressure arterial system results in more significant compression effects in the pleural cavity due to arterial blood loss [126].
As Deneuville et al. [121] and Yanagawa et al. [122] reported, penetrating injuries to the PA can involve laceration, transection, rupture, disruption, perforation, pseudoaneurysm, dissection, or fistula formation. Pseudoaneurysm rupture can be delayed for months.
The rapid initial assessment of trauma patients should follow the Advanced Trauma Life Support (ATLS) protocol established by the American College of Surgeons Committee on Trauma [127]. Diagnosis is often based on intraoperative findings, enhanced CT imaging, or pulmonary arteriography [122]. FAST (Focused Assessment with Sonography for Trauma) ultrasound can be used in the emergency setting to exclude hemothorax, pneumothorax, and cardiac complications. In unstable patients, transesophageal echocardiography (TEE) can also assess cardiac and valvular function [46]. There are no standardized guidelines for the management of pulmonary vessel injuries, and the approach depends on the type of injury, the patient’s condition, and institutional protocols.
Resuscitative thoracotomy should be performed urgently in patients with signs of life and shock due to acute massive blood loss. Access to the injured PA may require a median sternotomy for intrapericardial PA injuries or a postero-lateral thoracotomy for distal PA or PV injuries, often necessitating bypass facilities [120, 124]. If a major branch of the pulmonary vessels is involved, clamping the pulmonary hilum to exclude the PA and PV from circulation may be lifesaving.
If the PA cannot be repaired and must be ligated, the bronchial arteries typically supply blood to the affected lung parenchyma. Repair of the pulmonary veins with lateral venorrhaphy using continuous polypropylene suture [120] is preferred but technically challenging. If ligation of the PV is necessary, the involved lung parenchyma should also be resected to prevent infarction [86, 128]. In severe cases, segmentectomy, lobectomy, or rapid pneumonectomy may be the only viable options.
Pseudoaneurysms are often managed with endovascular techniques and coil embolization, whereas surgical management is typically reserved for cases at high risk of acute rupture [129].
Limitations
This study is subject to several limitations. Firstly, it is a narrative review, relying on previously published data, which may introduce publication bias. The heterogeneity of the studies included, particularly regarding diagnostic approaches and management strategies, poses challenges in synthesizing the findings into unified clinical guidelines. Additionally, the variability in imaging techniques, patient demographics, and injury severities across the studies reviewed may affect the generalizability of the conclusions. Another limitation is the lack of direct experimental data, as this review is based solely on secondary sources, limiting its ability to provide novel empirical insights into lung trauma biomechanics. Future research with more controlled experimental or clinical trials is necessary to validate the biomechanical models and improve treatment approaches for lung parenchymal injuries.
Conclusions
Lung parenchymal trauma, caused by various mechanisms such as blunt, penetrating, and blast injuries, presents significant clinical challenges due to its diverse impact on lung structure and function. The unique biomechanics of the lung, including its extracellular matrix, architecture, and response to mechanical forces, play a critical role in the extent and severity of injury. Early recognition of lung trauma through appropriate imaging and diagnostic tools, along with a comprehensive understanding of the injury mechanism, is crucial for optimizing treatment strategies. Tailored management approaches based on injury type and severity can significantly improve patient outcomes, particularly in reducing the risks of complications such as respiratory failure, infections, or long-term functional impairment. Future research focused on lung biomechanics and response to trauma will be essential in refining therapeutic interventions and enhancing recovery in thoracic trauma patients.