Introduction
Breast cancer is been recognised as the most frequent diagnosed cancer globally. It accounts for 12% of newly diagnosed cancer cases, followed by lung, colorectal, prostate, and gastric cancers. Regarding cancer-related mortality, breast cancer comes in fifth place with a rate of 6.9% after the lung (18%), colon (9.4%), liver (8%), and stomach cancer (7.7%) [1].The existence of malignant cells in blood vessels, lymphatic vessels, or both is known to be a potential prognostic indicator and is used as a biomarker to predict patient prognosis and outcome in cancer management. Lymphovascular invasion (LVI) has been associated with a poor prognosis in patients with malignancy [2], and it influences decisions regarding adjuvant chemotherapy in different cancers such as breast [3], colon [4], prostate [5], uterus [6], testis [7], and bladder [8]. Various new molecular technologies that study the genetic criteria of the tumour cells have been developed, and the results are utilized as predictive tools as well as prognostic markers for disease recurrence risk. These tests include oncotype DX recurrence score (ODX-RS) and MammaPrint70 [9]. The oncotype DX recurrence score is a molecular diagnostic assay that analyses the individual biological criteria of a breast cancer tumour by examining the activity of 21 genes in the tumour tissue. The Recurrence Score value is a number between 0 and 100 that can provide information about how likely a breast cancer is to recur within 10 years of diagnosis and, perhaps more importantly, the likelihood that the patient will benefit from chemotherapy.
This study aims to explore the association between ODX-RS and LVI status in oestrogen receptor-positive (ER +ve), human epidermal growth factor receptor–2–negative (HER2 –ve), low-burden axillary nodal disease early breast cancer, and the results impact on adjuvant chemotherapy indication.
Material and methods
Patients diagnosed with histologically confirmed ER +ve, HER2 –ve, low-burden axillary nodal disease early operable breast cancer at Basildon University and Thurrock University Hospital between 2017 and 2021 were enrolled (n = 102). The key inclusion criteria for the cohort were as follows: ER +ve/HER2 –ve and low-burden axillary nodal disease. The latter included node-negative (NO), micro-metastasis (Nmic), and early axillary disease (N1), early operable invasive breast cancer. The clinicopathological data available from the institutional database, including age, pathomorphological tumour subtype, tumour grade, size, presence of lympho-vascular invasion, lymph node status, type of surgery, and the information related to the offered adjuvant treatment (chemotherapy, hormonal therapy, and/or radiotherapy), were analysed. All the 102 patients had undergone adequate tumour surgical resection – either breast conservation surgery or mastectomy with/without reconstruction. The histopathology confirmed clear resection margins. Also, they had had either sentinel lymph node biopsy, axillary node sampling in NO disease, or axillary clearance in node-positive disease; all patients had no evidence of distant metastatic disease. The total number of the resected tumours was 107 as five patients had bilateral disease. Lymphovascular invasion was defined as malignant tumour cells present within a definite, endothelial-lined space (lymphatic and/or blood vessel) of peri-tumoral area. This was detected by the pathologists via immune-histochemistry studies (CD31 and CD34 for vascular and D2–40 for lymphatic). Lymphovascular invasion was considered to be positive (LVI +ve) if the tumour revealed focal or diffuse staining. The presence of the LVI was compared with the ODX-RS results, and the χ2 test was used to assess the relationship between the 2 variables. Also, the Ki-67 proliferation index (Ki-67-PI) of the cohort was measured using immune-histochemical analysis. This biomarker is a nuclear protein antigen best detected during the interphase of the cell cycle. It was calculated by using an immune-histochemistry assay. For Ki-67-PI categorization, the cut-off point used was 15%. For tumours with > 15% positive nuclei staining, the Ki-67-PI expression was classified as high, whereas those with < 15% were classified with low Ki-67 expression. The relationship between Ki-67-PI and LVI was analysed. The project was reviewed and approved by the Clinical Improvement Unit of the Basildon and Thurrock University Hospital NHS Trust.
Results
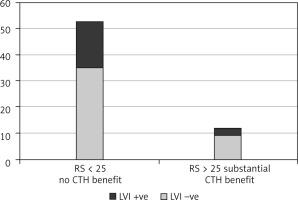
Five patients had bilateral disease, and 107 tumours were resected. The cohort age ranged between 34 and 75 years, median 55 ±10.8. Lymphovascular invasion was identified in 34 out of 107 resected tumours (32.6%); 13 patients were aged ≤ 50 years (Fig. 1), and 21 patients were > 50 years of age (Fig. 2). In the LVI +ve age group ≤ 50 years, 9 tumours had ODX-RS < 15 (69%), which is associated with no chemotherapy benefit; one tumour (8%) had ODX-RS between 15–20, which gives only ~ 1.6% chemotherapy benefit; and 2 tumours (15%) had ODX-RS 21–25, which gives up to 6.5% chemotherapy benefit. Only one tumour (8%) had RS > 25, and this is linked to a substantial chemotherapy benefit (Fig. 3). In the age group > 50 years, 21 tumours were LVI +ve, and 18 tumours had ODX-RS ≤ 25 (86%), which is associated with no chemotherapy benefit, whereas only 3 tumours (14%) had ODX-RS > 25, and this is linked to a substantial chemotherapy benefit (Fig. 4). For the total cohort, we used the value of 25 as an ODX-RS cut-off point. We applied the low ODX-RS category for results of ≤ 25, and high for ODX-RS >25. A low ODX-RS with positive LVI was seen in about 29% of the total cohort, and only 2.8% had high ODX-RS with positive LVI (p = 0.29). Also, the relationship between Ki-67-PI and LVI was analysed. The cut-off point for Ki-67-PI was 15%; low Ki-67 is > 15%, whereas high Ki-67 is ≥ 15%. A low Ki-67-PI and positive LVI were detected in 19% of the total cohort, and 13% had high Ki-67-PI and positive LVI (Table 1).
Fig. 1
Oncotype DX recurrence score in 13 breast cancer tumour with lympho-vascular invasion expression (age group ≤ 50 years)
CTH – chemotherapy, RS – oncotype DX recurrence score
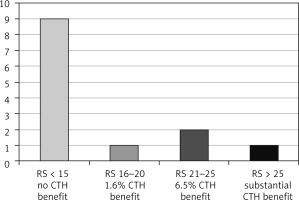
Fig. 2
Oncotype DX recurrence score in 21 breast cancer tumours with lympho-vascular invasion expression (age group > 50 years)
CTH – chemotherapy, LVI – lymphovascular invasion, RS – oncotype DX recurrence score
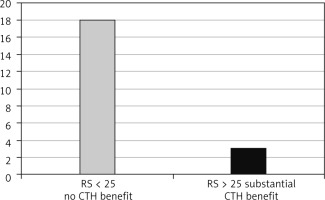
Fig. 3
The oncotype DX recurrence score and lymphovascular invasion expression in the age group ≤ 50 years
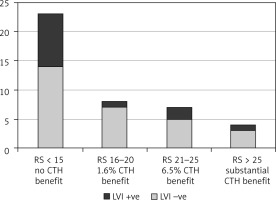
Fig. 4
The oncotype DX recurrence score and lymphovascular invasion expression in the age group > 50 years
Discussion
Recent reports have mentioned that the incidence of breast cancer has been increasing by 3.1% per annum, and this trend is expected to continue [10]. Despite the rising trend towards less radical breast surgical procedures and the use of breast conservation techniques, disease-free and overall survival rates from breast cancer have increased steadily over the last several years [11]. The indication of chemotherapy in ER +ve/HER2 –ve early breast cancer is traditionally based on factors providing prognostic information, such as age, fitness, tumour size/grade, axillary lymph node status, and lympho-vascular invasion. The risk of distant metastasis or disease recurrence may not simply be a function of large tumour size or temporal acquisition of metastatic ability, but this may exist as an inbuilt feature of the cancer from the very beginning of the neoplastic activity [12].
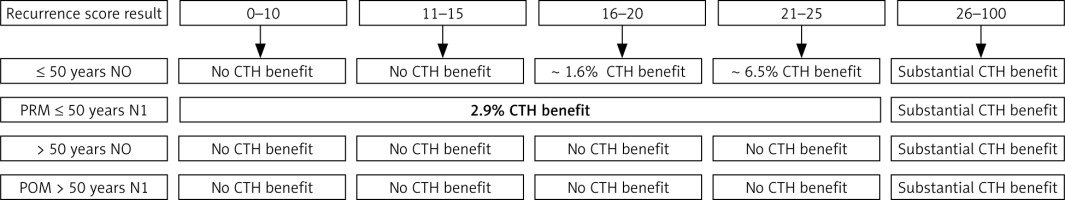
The term lympho-vascular invasion is applied when tumour cells are seen within a definite endothelial-lined space (blood vessels or lymphatics) within the breast tissue outside the main tumour of invasive breast carcinoma (peri-tumoral space); it is one of the crucial steps in cancer metastasis. Lympho-vascular invasion is not uniformly encountered in invasive breast cancer cases; it is reported in only about one-third of cases. It has been reported that LVI is associated with increased rates of other biological tumour markers such as high Ki-67-PI expression and axillary lymph node disease. Also, it is linked to a higher risk of systemic disease progression [13] and is considered as a negative effective factor for cancer relapse and as well as for survival in node-negative patients [14]. Lee et al. in 2006 published data related to a cohort of 2760 patients with node-negative, surgically treated, invasive breast cancer. The authors concluded that node-negative patients with lympho-vascular invasion had a higher mortality rate (53%) compared with patients with no lympho-vascular invasion (27%) [15, 16]. As mentioned above, the indication of adjuvant systemic therapy relied on disease clinic-pathological criteria; however, the genomic assay of the cancer tissue may provide additional information in tumour recurrence risk prediction and the extent of benefit associated with adjuvant chemotherapy [17]. Since the introduction of multi-gene assays guided treatment in early-stage breast cancer, such as the 21-gene recurrence score assay (oncotype DX) and 70-gene signature (MammaPrint), the indication of neoadjuvant chemotherapy in breast cancer has changed dramatically [18]. The oncotype DX recurrence score is a 21-gene assay that looks into the concurrent activity of 16 genes, in addition to 5 control genes, by polymerase chain reaction, using breast cancer tissue. The outcome is labelled a recurrence RS and presented as a value between 0 and 100. Also it provides an estimation of distant disease recurrence risk at 9 years when only hormonal manipulation therapy of selective oestrogen receptor modulators such as tamoxifen, or class I aromatase inhibitors such as Letrozole, are used as adjuvant therapy [19, 20]. The other information provided by ODX-RS is the expected benefit from both adjuvant chemotherapy and hormonal manipulation treatment [21–26] in reducing the breast cancer recurrence risk (Fig. 5). There is an additional value of ODX-RS: it is more sensitive for hormone receptor status assessment. The oncotype DX recurrence score has been validated to guide the use of adjuvant chemotherapy therapy in oestrogen ER +ve, HER2 –ve, NO or Nmic/low burden axillary disease (N1) early operable invasive breast cancer; this guideline has been recommended by NICE (National Institute for Health and Care Excellence-UK), NCCN (National Comprehensive Cancer Network-USA), and ASCO (American Society of Clinical Oncology). Some studies have attempted to correlate ODX-RS with different breast biological markers such as tumour pathomorphological subtype, tumour size, and grade. Because some researchers claim that the most closely related biomarker to ODX-RS is the Ki-67 proliferation index [27], we studied the LVI expression of each case in an attempt to identify any specific correlation to the ODX-RS. We found that, despite LVI detected in one-third of the cases, most of them had low ODX-RS. In contrast, those cases with LVI showed a greater percentage with higher levels of Ki-67 proliferative index. In the age group ≤ 50 years, with LVI +ve tumours, the rate of moderate/high ODX-RS was about 23%, which is linked to more than 6.5% chemotherapy benefit, whereas in the age group > 50 years, this figure was 14%. Because the association is not statistically significant, some authors recommend utilization of the fact that LVI has proven to be significantly associated with other well-known high-risk indicators, such as high tumour grade, larger tumour size, high, and heavier nodal disease, which may provide predictive and prognostic information beyond advanced molecular testing [28].
Fig. 5
Early ER +ve, HER2 –ve, low-burden axillary disease oncotype DX RS-guided treatment [21–26]
PRM – pre-menopausal, POM – post-menopausal, CTH – chemotherapy, NO – no lymph node metastasis, N1 – involvement of 1–3 lymph nodes
This study has its limitations: the relatively small sample size, and patients had heterogeneous cancer criteria because node positive disease patients were not included initially – this is due to changes in the recommendations. Also, the study was conducted in a single centre, and the follow-up outcome was not included due to the short period.
Conclusions
This study demonstrates the value of utilisation of ODX-RS during the adjuvant chemotherapy decision-making process in breast cancer management. In the targeted population, lympho-vascular invasion did not have a statistically significant impact on ODX-RS (p = 0.29). The study also emphasizes that ODX-RS as well as LVI could be used alongside other tumour biomarkers for management decision-making when appropriate.








