Multiple sclerosis (MS) is the most common immunemediated inflammatory demyelinating disease of the central nervous system. The immunomodulatory agents such as fingolimod used to treat the disease have been associated with several side effects, including severe infections. A mediastinal abscess with sternal osteomyelitis was never attributed to fingolimod therapy. It is usually associated with high morbidity and mortality and may follow a fulminant or subacute clinical course. Surgical exploration with drainage and debridement is the gold standard for effective treatment that protects against recurrence, mediastinitis and septic complications. Occasionally, when a mediastinal abscess coexists with sternal osteomyelitis, surgical debridement is followed by local negative pressure wound therapy or vacuum-assisted closure (VAC), prior to subsequent delayed pectoral flap or omental flap repair. Simultaneously, a meticulous study should be done to detect the origin of every mediastinal abscess. Esophageal ruptures, odontogenic infections, trauma complications and peritonsillar abscesses are the main causes. Rarely, hematogenous spread from other primary locations may lead to mediastinal abscess formation.
Herein, we present such an unusual case of an immunocompromised patient with hematogenous dissemination in the setting of sacrococcygeal osteomyelitis secondary to treatment with fingolimod.
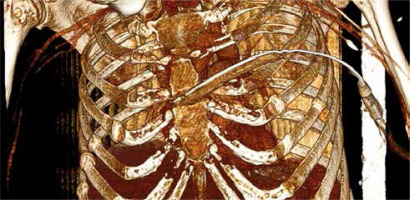
A 54-year-old female patient with relapsing-remitting multiple sclerosis was referred to us from another hospital after 48 h of hospitalization with a diagnosis of mediastinal abscess. The patient was severely disabled due to multiple sclerosis, bedridden for the last 3 months, and received immunomodulatory treatment with fingolimod 0.5 mg once daily. She presented with a large tender mass in the anterior thoracic wall, substernal pain, fever, night sweats, confusion, and tachypnea. On admission, the blood test results indicated a severe infection: white cell count 19500/μl, and C-reactive protein 18.4 mg/dl. Full body computed tomography (CT) revealed an extensive abscess of the anterior thoracic wall in contact with the large substernal mediastinal abscess and fragmentation of the middle sternal portion (Figures 1 A, B). A significant collection of air was noted inside both cavities. There were similar findings in the sacrococcygeal region with fluid and air collection, probably as a complication of decubitus ulcers.
Figure 1
A, B – Large mediastinal abscess drained through the fragmented sternum to the anterior thoracic wall
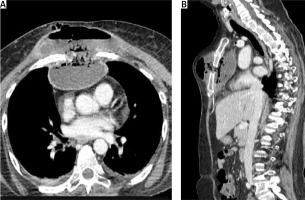
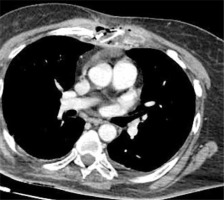
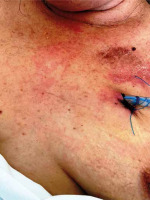
The patient was treated with broad-spectrum intravenous antibiotics during her previous hospitalization, which failed to improve her condition. Given her poor respiratory reserve (FEV1 = 0.6 l), a conservative approach with percutaneous drainage of the abscess was decided, if possible. Under local anesthesia, a small incision in the midclavicular line at the 2nd intercostal space was made. Using right-angle forceps, the subcutaneous tissue was dissected, and 1.5 l of frank pus was drained. Subsequently, a 32 Fr curved chest tube was inserted through the sternal bone defect inside the mediastinal abscess (Figure 2). In a repeated chest CT during the following days, it appeared that the abscess was completely drained and the cavity in the mediastinum was collapsed (Figure 3). The chest tube remained in the mediastinal cavity for the remainder of her hospital stay (Figure 4). Although no pathogen was isolated in routine cultures of blood and wound aspirates, antibiotics were upgraded to meropenem and vancomycin. The patient’s clinical condition and her septic parameters were drastically improved (WBC 8500/µl, CRP 0.36 mg/dl). However, her neurologic status deteriorated after 3 weeks due to temporary discontinuation of immunomodulatory medication and exacerbation of multiple sclerosis. Respiratory muscle weakness and respiratory failure occurred so she was intubated. Finally, the patient suffered cardiac arrest and died four weeks after admission although the sepsis had been successfully treated.
Fingolimod is a sphingosine analogue that modulates the sphingosine 1-phosphate receptor and thereby alters lymphocyte migration, resulting in sequestration of lymphocytes in lymph nodes [1]. Several randomized controlled trials showed that fingolimod reduced the relapse rates in relapsing-remitting multiple sclerosis [2]. On the other hand, fingolimod is associated with increased risk of cardiac arrhythmias, liver dysfunction, lung and thyroid complications, refractory headaches, encephalopathy, vasculopathy, tumor development and opportunistic infections [3]. This agent has also been associated with life-threatening herpes viral infections, lower respiratory tract infections and malignancies [4]. During treatment with fingolimod (and for 2 months after discontinuation), patients should be monitored for symptoms and signs of infection. Interruption of immunomodulatory treatment is often required to treat these serious infections and to identify and treat the causative microorganism. Unfortunately, cases of rebound syndromes and multiple sclerosis exacerbation occur within 24 weeks after discontinuation of fingolimod [5, 6].
Concomitant occurrence of osteomyelitis and mediastinal abscess during immunomodulatory treatment with fingolimod has never been reported. Osteomyelitis may result from direct inoculation of pathogens after trauma or surgery, from the adjacent propagation of an infectious process or from hematogenous spread to the bone during bacteremia [7]. In our case, decubitus ulcers had a decisive influence on sacrococcygeal osteomyelitis and then on the hematogenous spread to the mid-sternal portion. The illustration of the mediastinal abscess is impressive. This abscess actually formed a secondary abscess through the sternal bone defect to the subcutaneous tissue of the anterior thoracic wall. Spontaneous drainage into the subcutaneous tissue may have been responsible for avoiding a fulminant clinical course and mediastinitis. Thus, the abscess was treated conservatively without the need for emergency surgery, which was incompatible with the patient’s condition. Fortunately, the curved chest tube that was inserted through the sternal “gap” drained both the subcutaneous and the substernal abscesses. Percutaneous drainage of a mediastinal abscess, either CT-guided or by conventional chest tube as in our case, is mandatory to control infection. This is possible when the infection within the mediastinum is self-limiting, without evidence of extensive mediastinitis with air, multiple loculations in the mediastinal adipose tissue or pleural effusions. In this case, surgical drainage and debridement cannot be avoided. At the same time, the cause of mediastinitis must be addressed.
Hematogenous osteomyelitis is the most common form of osteomyelitis mainly in infants and children. Risk factors for hematogenous osteomyelitis in adults include endocarditis, presence of indwelling intravascular devices (vascular catheters, cardiovascular devices) or orthopedic hardware, injection drug use, hemodialysis, and sickle cell disease [8]. Most cases of acute hematogenous osteomyelitis are caused by Gram-positive bacteria, principally S. aureus. However, no pathogen is isolated here in routine cultures of blood and wound aspirates. This fact is also observed in up to one-half of cases, which are usually presumed to be caused by S. aureus and treated accordingly [9]. Furthermore, patients may present following clearance of bacteremia, so blood cultures are not always positive. Conventional therapy of a mediastinal abscess and sternal osteomyelitis include opening and debridement of the wound, serial packing, and finally primary closure of the wound. These surgical procedures were associated with an unacceptably high mortality rate, with rates reported between 20% and 40% in some series [10, 11]. High rates of treatment failure warranting additional surgical interventions are common [12]. The use of vacuum-assisted closure accelerates wound healing, and granulation has been proposed for the treatment of open wounds. An increase in local blood flow, decrease in tissue edema and bacterial load, and removal of stagnant fluid, necrotic debris and proteins impeding healing are all believed to promote wound healing with a vacuum-assisted device. Large series have demonstrated that vacuum-assisted closure is a useful tool, especially in post-sternotomy mediastinitis [13–16]. However, surgical debridement and application of vacuum-assisted closure demand multiple surgical interventions. To the best of our knowledge, successful drainage of a mediastinal abscess with a conventional chest tube through the fragmented sternum has never been described. Similarly, hematogenous sternal osteomyelitis during treatment with fingolimod has also never been reported.
The patient’s death is attributed to a relapse of multiple sclerosis with respiratory muscle weakness due to discontinuation of immunomodulatory treatment. Cases of rebound syndrome may occur within the first 24 weeks after stopping fingolimod treatment in patients with multiple sclerosis of variable severity and duration. In several cases, relapses have occurred despite the initiation of other disease-modifying therapies. Usually, rebound symptoms have involved back and extremity pain, confusion, diplopia, facial muscle spasms, fatigue, increased leg weakness, nausea, paraparesis and paresthesia [6]. Respiratory muscle weakness is extremely rare in relapsing multiple sclerosis. We cannot ascribe it to pulmonary embolism or lower respiratory tract infection as imaging tests and septic parameters were negative. Certainly, hypoxia and the acidotic milieu in addition to the arrhythmogenic effect of fingolimod played a crucial role in the development of resistant cardiac arrest.
In conclusion, relapsing-remitting multiple sclerosis is by far the most common type of MS and is characterized by clearly defined relapses. There is evidence from several randomized controlled trials that fingolimod reduces the relapse rate in this type of MS. However, its use is associated with severe adverse effects. Mediastinal abscess and sternal osteomyelitis are described as adverse effects of this immunomodulatory drug for the first time. Similarly, successful percutaneous and transsternal drainage of the abscess with a chest tube has never been reported. Although the sepsis was successfully treated, the patient died, reminding us that when immunodeficiency is combined with such devastating complications, catastrophic outcomes are to be expected.