Introduction
Mediastinitis seen after cardiac surgery is rare but is considered as a serious complication. It increases the length of postoperative hospital stay, mortality and morbidity. The incidence after median sternotomy is in the range of 1–2% [1]. Mortality was in the range of 2–8%. However, the mortality rate of mediastinitis due to comorbid conditions reaches 14% [2].
According to the Centers for Disease Control and Prevention (CDC) definition of surveillance for specific infections, mediastinitis must meet at least one of the following criteria [3]:
The patient has pathogen(s) identified from mediastinal tissue or fluid by a culture or non-culture based microbiologic testing method which is performed for the purposes of clinical diagnosis or treatment.
The patient has evidence of mediastinitis on gross anatomic or histopathologic examination.
The patient has at least one of the following signs or symptoms:
Fever (> 38.0°C),
Chest pain (with no other recognized cause),
Sternal dehiscence,
And at least one of the following:
Purulent drainage from the mediastinal area,
Mediastinal widening in imaging test.
Although mediastinitis is a surgical site infection, risk factors need to be modified to prevent this complication. The results obtained in the studies conducted for this purpose vary according to the demographic data and the designs of the studies. Many dependent and independent risk factors have been identified [1, 2, 4–10].
Mediastinitis treatment covers a wide range from simple extended antibiotic treatment to full sternectomy and major plastic procedures [11]. Conventional treatment includes repeated wound irrigations, debridements and various sternal reconstruction techniques with antibiotics and disinfectants [12–14]. The high mortality and morbidity rates of conventional mediastinitis therapies have led to an alternative treatment over time. The superiority of vacuum-assisted closure (VAC) in mediastinitis treatment has been demonstrated by studies and meta-analysis over time [15, 16].
VAC provides simultaneous chest stabilization by isolating the wound while continuously removing the exudate from it. It induces granulation tissue formation by providing a constantly moist environment and induces wound healing by increasing blood flow from surrounding tissue [17]. Reducing tissue edema also regulates capillary permeability and osmolarity by preventing compartment syndrome occurring at the wound edges [18]. Also, studies report decreasing bacterial load within the wound [19].
VAC treatment can be repeated in mediastinitis cases until the sternal reconstruction stage. This reconstruction may be possible with sternum re-wiring or muscle skin flaps [14]. It has been observed that VAC treatment decreases mortality and recurrent mediastinitis when applied as a bridge therapy to flap surgery [20].
Aim
In our study, we aimed to determine risk factors according to our demographic structure and examine our VAC treatment results.
Material and methods
The study was approved by the Ethical Committee of Health Sciences University Mehmet Akif Ersoy Training and Research Hospital according to the decision dated 08/06/2018 and numbered 2018/26.
In this study, 9160 cases of patients who underwent cardiac surgery with median sternotomy between January 2010 and December 2017 in Istanbul Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital, Department of Cardiovascular Surgery were reviewed retrospectively. In this period, 427 (69.3%) (n = 296) male and 30.7% (n = 131) female patients with mean age 60.81 ±10.47 were included in the study. Information about the cases was obtained from hospital archive files and electronic medical record system.
Patient selection
Our study was divided into two groups. The mediastinitis group included 127 patients diagnosed with mediastinitis who were treated with the VAC technique. By evaluating risk factor analysis data in similar literature, the sample size was calculated with 80% power and 5% type-1 error. The control group was chosen among eligible patients with no evidence of surgical site infection, and who had not undergone minimally invasive open-heart surgery by standard surveillance and chart review. The control group was matched 2 : 1 per case and identified by using a random number table using ±5 sorted by surgery date (within the same year), gender and age.
Superficial surgical site infections, patients under 18 years of age who had undergone cardiac surgery, patients who had undergone minimally invasive open-heart surgery, patients who had undergone cardiac surgery in other centers and then received mediastinitis treatment in our center were excluded from our study.
Between the two groups preoperative (age, gender, body surface area (BSA), ejection fraction (EF), smoking, diabetes mellitus (DM), hypertension, chronic obstructive pulmonary disease, presence of other comorbid systemic diseases, hematocrit (Htc), hemoglobin (Hb), white blood cell count, platelets, calcium, mean platelet volume (MPV), C-reactive protein (CRP), sedimentation, glycated hemoglobin (HbA1c), cholesterol, and vitamin B12 levels), intraoperative (type of surgery, surgical timing, presence of redo surgery, internal thoracic artery (ITA), bilateral ITA (BITA) use, total pump time (TPT), aortic cross clamp time, bone-wax use, EuroSCORE) and postoperative (Inotropic support use, intra-aortic balloon pump (IABP), intensive care unit stay, blood product use, amount of drainage, length of hospital stay, presence of mortality) parameters were compared in terms of significant difference. The effects of perioperative parameters on the mediastinitis treatment were evaluated in patients diagnosed with mediastinitis.
Statistical analysis
Statistical analysis was performed to determine the independent risk factors in the development of mediastinitis with the parameter data that differed significantly between the two groups, and to show whether it was effective with respect to VAC treatment time and mortality.
Number Cruncher Statistical System (NCSS) 2007 (Kaysville, Utah, USA) was used for statistical analysis. Descriptive statistical methods (mean, standard deviation, median, first quarter, third quarter, frequency, percentage, minimum, maximum) were used to evaluate the study data. The suitability of the quantitative data for normal distribution was tested by the Shapiro-Wilk test and graphical analysis. The independent groups t test was used for comparison between two groups of quantitative variables showing normal distribution, and the Mann-Whitney U test was used for comparison between two groups of quantitative variables that did not show normal distribution. Pearson c2 test and Fisher’s exact test were used to compare qualitative data. Spearman correlation analysis was used to evaluate the relationships between quantitative variables. Receiver operating characteristic (ROC) analysis was used to determine the limit values for the continuous variables that affect mediastinitis. Independent risk factors and estimated relative risks (odds ratio) were determined by logarithmic regression analysis. Linear regression analysis (backward) was used to determine the factors affecting the duration of VAC treatment. Statistical significance was accepted as p < 0.05.
Results
BSA values, diabetes mellitus incidence, urea, creatinine, CRP, sedimentation, HbA1c value, and preoperative hospital stay were significantly higher in mediastinitis (+) cases compared to mediastinitis (–) cases (p < 0.01).
EF values, albumin, MPV, and HDL values of patients with mediastinitis (+) were significantly lower than those with mediastinitis (–) (p = 0.001; p < 0.01).
There was a statistically significant difference in the variety of surgical operations in terms of the incidence of mediastinitis (p < 0.05). When we compare separately to see which of these operations make a significant difference, isolated coronary artery bypass graft (CABG), isolated valve surgery, valve surgery with additional procedures, and emergency surgery were associated with a higher rate of mediastinitis (p < 0.019; p < 0.002; p < 0.048, p < 0.028 respectively).
Among the operative and postoperative parameters, emergency surgery rate, left internal thoracic artery (LITA) use rate, BITA use rate, TPT durations, IABP use rate, ES, fresh frozen plasma (FFP), apheresis platelet usage amounts, postoperative drainage amounts, and postoperative hospital stay were found to be statistically significantly higher in the mediastinitis group than in the control group (Table I).
Table I
Peroperative parameters
| Preoperative parameters | Mediastinitis group | Control group | P-value | ||
|---|---|---|---|---|---|
| Age | Mean ± SD | [years] | 62.16 ±9.01 | 60.23 ±10.99 | a0.082 |
| BSA | Mean ± SD | [kg/m<sup>2</sup>] | 1.94 ±0.16 | 1.88 ±0.18 | a0.006** |
| EF | Median (min.–max.) | (%) | 55 (30–65) | 60 (30–70) | c0.001** |
| Smoking | n (%) | Yes No | 52 (42.3) 71 (57.7) | 109 (36.7) 188 (63.3) | b0.285 |
| Smoking amount | Median (min.–max.) | [package/year] | 12 (0–70) | 15 (0–150) | c0.557 |
| Gender | n (%) | Male Female | 83 (65.4) 44 (34.6) | 209 (69.7) 91 (30.3) | b0.381 |
| DM | n (%) | No Yes | 48 (37.8) 79 (62.2) | 156 (52.2) 143 (47.8) | b0.007** |
| Hypertension | n (%) | No Yes | 32 (25.2) 95 (74.8) | 100 (33.4) 199 (66.6) | b0.092 |
| COPD | n (%) | No Yes | 62 (48.8) 65 (51.2) | 152 (50.8) 147 (49.2) | b0.703 |
| Additional disease | n (%) | No Yes | 81 (63.8) 46 (36.2) | 216 (72.2) 83 (27.8) | b0.082 |
| Preoperative hospitalization duration | Median (min.–max.) | [day] | 1 (1–30) | 1 (0–31) | c0.001** |
| Preoperative laboratory parameters | Mediastinitis group | Control group | P-value | ||
| HTC | Mean ± SD | (%) | 38.71 ±5.3 | 39.71 ±4.72 | a0.054 |
| HB | Mean ± SD | [g/dl] | 12.98 ±2.14 | 13.24 ±1.57 | a0.222 |
| WBC | Mean ± SD | [× 109/l] | 8.48 ±2.9 | 8.49 ±2.37 | a0.954 |
| Thrombocyte count | Mean ± SD | [× 109/l] | 252.67 ±75.54 | 266.79 ±84.56 | a0.105 |
| Urea | Median (min.–max.) | [mg/dl] | 19 (10–112) | 16 (7–60) | c0.001** |
| Creatinine | Median (min.–max.) | [mg/dl] | 1.0 (0.4–13.2) | 0.84 (0.48–4.21) | c0.001** |
| Albumin | Mean ± SD | [g/dl] | 3.42 ±0.7 | 4.38 ±0.48 | a0.001** |
| Calcium | Mean ± SD | [mmol/l] | 9.01 ±0.99 | 8.95 ±0.56 | a0.543 |
| MPV | Mean ± SD | [fl] | 9.03 ±1.3 | 10.89 ±0.97 | a0.001** |
| CRP | Median (min.–max.) | [mg/l] | 4.6 (1–70) | 3.19 (1–99) | c0.018* |
| Sedimentation | Mean ± SD | [mm/h] | 36.03 ±26.26 | 23.16 ±14.65 | a0.001** |
| HbA1C | Mean ± SD | (%) | 8.03 ±1.87 | 6.85 ±1.89 | a0.001** |
| Total cholesterol | Mean ± SD | [mg/dl] | 187.28 ±66.67 | 188.53 ±54.05 | a0.845 |
| LDL | Mean ± SD | [mg/dl] | 114.36 ±43.75 | 111.06 ±42.47 | a0.487 |
| HDL | Mean ± SD | [mg/dl] | 40.93 ±11.05 | 44.91 ±11.23 | a0.001** |
| Vitamin B12 | Median (min.–max.) | [pg/ml] | 456 (176–1613) | 303 (86–1299) | c0.049* |
| Intraoperative parametersΦ | Mediastinitis group | Control group | P-value | ||
| Isolated CABG | n (%) | 98 (77.2) | 197 (65.7) | b0.019* | |
| CABG + valve surgery | n (%) | 15 (11.8) | 23 (7.7) | b0.170 | |
| CABG + other surgery | n (%) | 1 (0.8) | 4 (1.3) | d1.000 | |
| Isolated valve surgery | n (%) | 5 (3.9) | 43 (14.3) | b0.002** | |
| Valve + other surgery | n (%) | 2 (1.6) | 18 (6) | b0.048* | |
| CABG + valve + other surgery | n (%) | 4 (3.1) | 4 (1.3) | d0.245 | |
| Other surgery | n (%) | 2 (1.6) | 11 (3.7) | d0.413 | |
| EuroSCORE | Median (min.–max.) | 2.2 (0.55–32.1) | 1.71 (0.5–83.9) | c0.206 | |
| Intraoperative parameters | Mediastinitis group | Control group | P-value | ||
| Emergency surgery | n (%) | No Yes | 112 (88.2) 15 (11.8) | 283 (94.3) 17 (5.7) | b0.028* |
| TPT | Median (min.–max.) | [min] | 90 (0–467) | 91.5 (0–360) | c0.004** |
| ACCT | Median (min.–max.) | [min] | 48 (0–244) | 49 (0–1000) | c0.311 |
| Redo surgery | n (%) | No Yes | 123 (96.9) 4 (3.1) | 284 (95.0) 15 (5.0) | b0.393 |
| LITA use | n (%) | No Yes | 23 (18.1) 104 (81.9) | 113 (37.8) 186 (62.2) | b0.001** |
| BITA use | n (%) | No Yes | 120 (94.5) 7 (5.5) | 298 (99.7) 1 (0.3) | d0.001** |
| Bone wax use | n (%) | No Yes | 83 (65.4) 44 (34.6) | 219 (73.2) 80 (26.8) | b0.101 |
| Postoperative parameters | Mediastinitis group | Control group | P-value | ||
| IABP use | n (%) | No Yes | 119 (93.7) 8 (6.3) | 297 (99.3) 2 (0.7) | d0.001** |
| Inotropic use | n (%) | No Yes | 80 (63.0) 47 (37.0) | 169 (56.5) 130 (43.5) | b0.215 |
| Mechanical ventilation duration | Median (min.–max.) | [h] | 12 (10–2880) | 12 (12–960) | c0.584 |
| ICU duration | Median (min.–max.) | [h] | 24 (22–2976) | 24 (24–1200) | c0.549 |
| ES use | Median (min.–max.) | [U] | 4 (0–27) | 2 (0–30) | c0.001** |
| FFP use | Median (min.–max.) | [U] | 3 (0–35) | 2 (0–100) | c0.001** |
| Apheresis thrombocyte use cases | n (%) | No Yes | 79 (62.2) 48 (37.8) | 257 (86.0) 42 (14.0) | b0.001** |
| Total drainage | Median (min.–max.) | [ml] | 650 (200–3300) | 350 (100–2000) | c0.001** |
| Postoperative hospitalization duration | Median (min.–max.) | [day] | 8 (2–185) | 6 (0–71) | c0.001** |
| Mortality | n (%) | No Yes | 100 (78.7) 27 (21.3) | 286 (95.3) 14 (4.7) | b0.001** |
Other surgical intervention: aortic surgery, ventricular and atrial septal defect closure, intracardiac mass excision, widening of the left ventricular outflow tract, left ventricular aneurysm repair, pulmonary embolectomy, pericardiectomy, epicardial lead placement. ACCT – aortic cross clamp time, BITA – bilateral internal thoracic artery, BSA – body surface area, CABG – coronary artery bypass graft surgery, COPD – chronic obstructive pulmonary disease, CRP – C-reactive protein, DM – diabetes mellitus, EF – ejection fraction, ES – erythrocyte suspension, FFP – fresh frozen plasma, HB – hemoglobin, HDL – high density lipoprotein, HTC – hematocrit, IABP – internal aortic balloon pump, ICU – intensive care unit, LDL – low density lipoprotein, LITA – left internal thoracic artery, MPV – mean platelet volume, PLT – platelets, TPT – total pump time, WBC – white blood cells.
The results of ROC analysis showed that preoperative laboratory values of albumin and HbA1c had diagnostic value in predicting the development of mediastinitis after cardiac surgery (GA: 95%, p < 0.001) (Table II).
Table II
Parameters with diagnostic value for mediastinitis
| Parameter | Limitvalue | Sensitivity(%) | Specificity(%) | Positivepredictivevalue (%) | Negativepredictivevalue (%) |
|---|---|---|---|---|---|
| Albumin[g/dl] | 4.1 | 74 | 81 | 77 | 22 |
| HbA1c (%) | 7.65 | 70 | 81 | 55 | 23 |
As a result of regression analysis, independent risk factors for the development of mediastinitis after cardiac surgery were found to be DM, BITA use and IABP use (Table III). In the statistical analysis performed for the effect of the presence of DM on the development of mediastinitis in patients using BITA, no significant difference was found.
Table III
Independent risk factors for mediastinitis
| Risk factors | RR (95% GA)* | P-value |
|---|---|---|
| DM | 2.38 (1.12–5.04) | 0.02 |
| BITA use | 10.37 (1.02–105.92) | 0.05 |
| IABP use | 9.10 (1.20–63.74) | 0.03 |
VAC treatment parameters were evaluated (Table IV). Parameters affecting the duration of treatment were analyzed. In univariate analysis, Htc, Hb, calcium, sedimentation, HbA1c, HDL, EuroSCORE values and female gender, BITA use, inotropic use, and microbiological evaluation results were found to be independent factors affecting the duration of VAC treatment. Linear regression (backward) analysis was used to study the multivariate effects. In the regression analysis, it was found that the presence of microorganism growth at wound culture had a significant effect on the VAC treatment duration.
Table IV
VAC treatment parameters
Regression model: VAC treatment duration (days)= 13.79 + 23.31 × (Culture (+)).
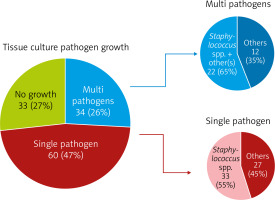
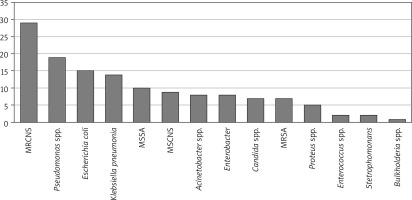
While there was no growth in tissue culture for 33 of the cases who received mediastinitis treatment, a single microorganism was observed in 60 and multiple microorganisms were observed in 34. Although a wide variety of microorganism growth was observed in the cases, mostly Staphylococcus species bacterial growth was detected (Figures 1, 2).
In the group treated for mediastinitis and in the logarithmic regression analysis no independent factor increasing mortality was detected.
Discussion
Modern surgical practices have significantly reduced the rate of wound infections. However, despite the improvement in surgical techniques and antimicrobial prophylaxis, there was no decrease in infection rates towards the end of the twentieth century [21]. The clinical and financial consequences of surgical site infections are quite severe for the patient, clinician, and hospital. Higher infection rates mean higher morbidity and mortality as well as higher costs for the hospital, patient, and community. Mediastinitis after cardiac surgery is a very serious complication that increases hospital costs, hospitalization time and mortality. Fortunately, mediastinitis is rare, occurring only in 1–3% of patients undergoing cardiac surgery [2, 7, 9, 10, 22–24]. In our center, the incidence of mediastinitis after cardiac surgery with median sternotomy was calculated as 1.4%. In a multicenter prospective study, the median duration of mediastinitis after the first surgical procedure was 20 days, and 65% of the patients were diagnosed with mediastinitis at re-admission after discharge [25]. In our study, we found these values to be similar, 18 days and 65% (82 of 127 cases).
Prevention of wound infections requires antisepsis, asepsis, perioperative antimicrobial prophylaxis, good surgical technique, preoperative identification of patients at risk and additional individual protective measures. Numerous large studies have identified several risk factors for mediastinitis [1, 2, 4–10]. These include obesity, diabetes mellitus, smoking, bilateral internal thoracic artery use, graft material, prolonged mechanical ventilation and redo surgery hospitalization period. However, the study results are often contradictory. Retrospective or prospective study design, study population size, and wound infection definitions are different. These inconsistencies make it difficult to compare findings. Demographic structures also show regional variations (race, season, etc.), which affects the results.
Since wound infections are one of the most feared complications after cardiac surgery, it is important to keep the operating room contamination at the lowest possible level. Studies in cardiac operations have shown that improving the surgical environment, refining surgical and operating room operations, and increasing awareness of the dangers of infection among staff can reduce the rate of infection [26]. It has also been shown by the infection committee that these rates can be reduced by close monitoring of hospital infections and applying necessary interventions [24].
There is a wide range of treatment when considering surgical site infections varying from non-invasive method options to those that require long hospitalization and need repeated interventions. The traditional treatment includes a broad spectrum of antibiotics with/without open dressings and/or closed irrigations. Studies compare traditional/conventional methods with VAC treatment in patients diagnosed with mediastinitis [12, 13, 15, 16, 27–29].
In many mediastinitis risk factor analysis studies, diabetes has been identified as an important risk factor [1, 5, 7]. DM is responsible for end organ damage and is a common disease that causes delays in wound healing. Especially uncontrolled blood sugar levels and insulin dependent DM increase the frequency of complications of the disease. In our study, we demonstrated that high values of HbA1c, which is an indicator of long-term blood sugar regulation, is a risk factor for complications, as well as a risk factor for diabetes.
Good nutritional status is essential for the process of wound healing. Ignoring the nutritional status can jeopardize the patient’s ability to recover and then prolong the stages of wound healing. Protein deficiency contributes to reduced collagen formation and wound separation, therefore resulting in poor healing rates. Nutrition holistic assessment and early detection of malnutrition support effective wound healing. There is a strong relationship between nutritional status and serum albumin value [30, 31]. In our study, we found that albumin values were significantly low in patients with mediastinitis. However, vitamin complexes are also known to be effective in wound healing [32]. In our study, we found that high vitamin B12 values were a significant variable in predicting mediastinitis but not an independent risk factor. In our ROC analysis which studied albumin value, we found that mediastinitis is frequently seen in cases below the limit value of 4.1 g/dl and poses a risk for the development of mediastinitis.
Although good surgical technique can reduce surgical site infection, the frequency of wound infection remains the same due to the increasingly complex operation procedures. In studies conducted, most cases of mediastinitis occur in patients who have undergone CABG. The fact that CABG is the most frequently performed process in cardiac surgery also leads to this result.
Sternum nutrition is provided by the branches that the intercostal arteries and internal thoracic artery give to the sternal bone [33, 34]. Using the left and/or right internal thoracic artery as a graft during CABG operation affects sternum feeding. Boerger, Ridderstolpe, Diez et al. [2, 5–7] and a number of other studies concluded that the use of the bilateral internal thoracic artery is a risk factor for the development of mediastinitis. In our study, we found that cases with isolated CABG, isolated valve surgery and valve surgery combined with other procedures were a significant variable in predicting mediastinitis, but not an independent risk factor.
Perrault et al. [1] found that the risk of mediastinal infection increased 5.81 times in ventricular assist device and transplantation cases. In our study, we found that the use of IABP, which is an intravascular mechanical support device, was an independent risk factor for the development of mediastinitis. In both VAD and IABP systems, there is a foreign substance inside the body and an extension outside the body. This connection with the external environment may have created a risk of infection from the outside to the inside. Mortality was significantly higher in mediastinitis cases than in controls. We think that it is an expected result because the mortality is multifactorial and mediastinitis risk factors have an effect on mortality and morbidity.
In studies conducted by Sjögren et al. [14, 29], it was emphasized that risk factors for the development of mediastinitis and the mortality of conventional treatment reached 45%. Although the negative effects of conventional treatment are not clear, it is believed that the progression of serious systemic infection with septic episodes due to infective treatment causes irreversible end organ damage. VAC treatment can be used as a single treatment method as well as a bridge treatment to final cure, until re-wiring.
Fuchs et al. [27] found that treatment with VAC therapy shortened hospitalization compared to conventional treatment. In their study, Petzina et al. [15] compared VAC and conventional therapy in patients with mediastinitis. They concluded that negative pressure wound therapy reduces mortality and sternal reinfection rates. Vos et al. [28] reported that intensive care follow-up was shorter and survival was longer in patients undergoing VAC treatment. They also demonstrated that VAC treatment not only reduces mortality, but also increases sternal stability, providing patient comfort by increasing patient mobilization.
Risnes et al. [12] compared VAC therapy and the conventional method in mediastinitis treatment and found that reinfection and treatment failure were more common in conventional treatment. The study of Tarzia et al. [13] showed that the average cost of patients treated with the conventional method was significantly higher than those treated with VAC therapy.
Onan et al. [35] demonstrated that mediastinitis is a serious postoperative condition in pediatric cardiac surgery patients and cannot be treated with conventional methods, so VAC therapy is an effective way to successfully treat the condition.
Studies show the superiority of VAC treatment in terms of survival, treatment success and cost when compared to conventional methods [12, 13, 15, 16, 27–29]. In our center, we used only VAC therapy instead of traditional methods in mediastinitis treatment. The debridement and resection procedures required for the wound sites were performed under operating room conditions, followed by VAC therapy. As a result of clinical and laboratory evaluations, VAC treatment was terminated, and the wound was closed with appropriate methods such as rewiring and muscle skin flap. Unfortunately, we were unable to compare conventional methods with VAC, and this is the limitation of our study. Mediastinitis after cardiac surgery was treated with conventional methods for a long time. As a result of the developments in wound care and treatment in the last 20 years, new pages have been opened in the treatment of mediastinitis.
However, we did not find a study examining whether the factors that increase the risk of occurrence of the disease affect the treatment. The effects of the parameters collected on the mortality and duration of treatment were analyzed.
Biofilm layers can develop in all open wounds. In chronic wounds, biofilms can prevent healing, prolong healing time, and play a role in causing chronic inflammation and increasing the risk of infection [36]. Biofilm formation causes delay in recovery by excessive inflammation, nitric oxide, inflammatory cytokines, excessive and long-term stimulation of free radicals and activation of immune complexes and complement [37]. In our study, the linear regression showed that growth in tissue cultures prolongs the duration of VAC treatment. Also, in the logarithmic regression analysis, no independent risk factor affecting mortality was detected. Rahim et al. [38] reported that the most common microorganisms seen in chronic wounds in their study were Staphylococcus, Pseudomonas, Peptoniphilus, Enterobacter, Stenotrophomonas, Finegoldia, and Serratia. In our study, the variety of microorganism growth was similar to the literature and it was noted that positive wound tissue culture has a significant effect on the VAC treatment duration.
Conclusions
We believe that the establishment of peroperative blood glucose regulation, patient selection to use BITA grafts, and maximum attention to sepsis and antisepsis rules in patients who need mechanical support devices such as IABP, will significantly reduce the development of mediastinitis. Also, an albumin value less than 4.1 g/dl, and a HbA1c level higher than 7.65% are in the risk group for mediastinitis. We think that VAC therapy is a successful and safe treatment of mediastinitis and should be used more widely.
The main goal is to reduce the incidence of the disease and prevent morbidity and mortality. Increasing the number of studies and cases included is important in order to standardize risk factors, establish and develop scoring systems, and make treatments more effective. Therefore, understanding the etiology, reducing wound infections and applying the most effective treatment require a multidisciplinary and selfless approach and effort.