Introduction
In 2019, several cases of pneumonia were reported in Wuhan, China. The pneumonia was later diagnosed to be caused by a novel Coronavirus Disease-19 infection (COVID-19) by the World Health Organization (WHO). In February 2020, after the spread of COVID-19 infection outside China, the COVID-19 infection outbreak was declared by the WHO [1].
Previous studies reported an abnormal coagulation (i.e. hypercoagulation status), affecting individuals infected with COVID-19 infection [2, 3]. The prevalence of venous thrombo-embolism has increased in critically ill COVID-19 infection individuals, despite prophylactic anticoagulants [4, 5]. Elevated D-dimer was reported in critically ill COVID-19 individuals; therefore, prophylactic (thromboprophylaxis) or therapeutic anticoagulants were recommended during the COVID-19 infection [6].
The causes of abnormal coagulation (i.e. hyper- coagulation status) during the COVID-19 infection are not clearly understood, but it can be explained by the endothelial injury, and immobilization of the critically ill COVID-19 infected individuals [7–9].
Endothelial injury can occur following invasion of the endothelial cells by severe acute respiratory syndrome coronavirus-2 [SARS-CoV-2 (COVID-19)] or following activation of the individual’s immune inflammatory response with subsequent elevated interleukin-6 (IL-6) and markers of complement activation (i.e. C5b-9) [3, 7].
Moreover, several changes in the circulating prothrombotic factors have been reported in critically ill COVID-19 infection individuals such as elevated factor VIII and fibrinogen, circulating prothrombotic microparticles, and neutrophil extracellular traps [10].
All hospitalized COVID-19 individuals should receive thromboprophylaxis unless contraindicated according to guidelines from the American Society of Haematology, and preferably low molecular weight heparin [11].
Uterine bleeding is less common in women infected with COVID-19 infection, but it may occur, especially after the use of thrombo-prophylaxis or therapeutic anticoagulants [12, 13]. Abnormal uterine bleeding (AUB) is one of the common complications of anticoagulants; it causes iron deficiency anaemia, [14], and occurs in 70% of reproductive-age women receiving anticoagulants [15].
Recently a cross-sectional study found that COVID-19 infection could affect the menstrual flow pattern, and it recommended future studies to confirm this finding [16].
Therefore, the current study was designed to detect the menstrual changes after the thrombo-prophylaxis or anticoagulants used during the COVID-19 infection.
Material and methods
A total of 176 women infected with COVID-19 infection were included in this questionnaire-based retrospective study, which was conducted from January 2021 to March 2021 at Ain Shams University, Maternity Hospital (ASMH), after approval of the study protocol by the local Institutional Review Board.
Participants were included in this questionnaire-based retrospective study, after giving their consent, following the Helsinki regulation.
COVID-19 infection was diagnosed by nasopharyngeal swab and chest CT (computerized tomography), with/without clinical findings (i.e. anosmia, dyspnoea, dry cough, and/or gastrointestinal troubles).
Inclusion criteria include reproductive-age women (> 20 and < 40 years old), who were infected with of COVID-19, regardless of whether they received thromboprophylaxis or therapeutic anticoagulants during the COVID-19 infection.
Exclusion criteria include women < 20 or > 40 years old, menopausal women, women diagnosed with AUB before the COVID-19 infection, or received thromboprophylaxis or anticoagulants within 3–6 months before this study, and refused to participate.
The PALM (defines structural AUB: polyp, adenomyosis, leiomyoma, and malignancy), and COEIN (defines functional AUB: coagulopathy, ovulatory, endometrial, iatrogenic, and non-classified) classification of AUB was used to diagnose AUB [15]. A pictorial chart was used to estimate the menstrual blow flow [15].
Participants were asked to complete an online questionnaire (available at: https://docs.google.com/forms/d/e/1FAIpQLScjV2JA35_QdAhWqaoQsPPyfR33HXpHVc0mv1hDlVFh9XQd2Q/viewform?vc=0&c=0&w=1&flr=0&usp=mail_form_link), after giving informed consent.
The online questionnaire included the participants’ demographic characteristics (i.e. age, marital condition, education, socio-economic level, job, and special habits); obstetric history (i.e. number of deliveries, mode of deliveries, and number of miscarriages); menstrual history (i.e. age of menarche, menstrual regularity, duration of flow, number of pads used/day, associated symptoms, and average cycle length); contraceptive history (i.e. last contraceptive method used, when started, and when stopped); chronic medication history, thrombo-embolic disorders, and/or bleeding disorders; COVID-19 infection (i.e. date and method of COVID-19 diagnosis, symptoms, history of hospital, and/or intensive care unit [ICU] admission); abnormal laboratory findings; thromboprophylaxis or the anticoagulants given; and menstrual changes after COVID-19 infection (i.e. menstrual flow days, number of pads used/day, menstrual regularity, and its duration).
The collected data were analysed using the χ2 test to detect the menstrual changes after the thromboprophylaxis or anticoagulants used during the COVID-19 infection.
Sample size and statistical analysis
The sample size was calculated using G Power software version 3.1.9.7 [17], and χ2 test. The qualitative variables were analysed using the χ2 test to detect the menstrual changes after the thromboprophylaxis or anticoagulants used during the COVID-19 infection. P < 0.05 was considered significant.
Results
A total of 176 women infected with COVID-19 were included in this questionnaire-based retrospective study to detect menstrual changes after the use of thromboprophylaxis or anticoagulants during COVID-19 infection.
The characteristics of the studied participants are listed in Table 1. Table 2 shows the studied participants’ menstrual patterns and the last contraceptive methods used before COVID-19 infection.
Table 1
Demographic characteristics of the studied participants
Table 2
The studied participants’ menstrual pattern and the contraceptive methods used before COVID-19 infection
The most common participants’ symptoms during the COVID-19 infection were body aches and myalgia (21.6%), followed by fever (20.5%), and loss of taste and smell (17%).
About 26.1% (46/176) the studied women were hospitalized, 4.5% (8/176) of them were admitted to the ICU, and C-reactive protein (CRP) and lactate dehydrogenase (LDH) were the most common abnormal laboratory findings observed among the studied participants [31.3% (55/176) and 30.1% (53/176), respectively] (Table 3).
Table 3
Hospital and ICU admissions, abnormal laboratory findings, thromboprophylaxis, and therapeutic anticoagulant used during COVID-19 infection
Regarding the thromboprophylaxis or the anticoagulant used during the COVID-19 infection, 32.4% (57/176) of the studied participants did not receive any thromboprophylaxis and/or anticoagulants, while 43.2% (76/176) of them received thromboprophylaxis, and 24.4% (43/176) received therapeutic anticoagulant during the COVID-19 infection (Table 3).
The factor Xa inhibitor, (Xalerto®, Janssen Pharma., USA or Rivarospire®, Atico Pharm., Egypt) were used as thromboprophylaxis in 38.2% (29/76) of the studied participants during the COVID-19 infection, and acetylsalicylic acid (antiplatelet), (Aspocid®, CID Pharm., Egypt) was used as thromboprophylaxis in 30.3% (23/76) of them. Rivarospire® was used as a therapeutic anticoagulant in 41.9% (18/43) of the studied participants during the COVID-19 infection (Table 3).
Menstrual changes during the COVID-19 infection
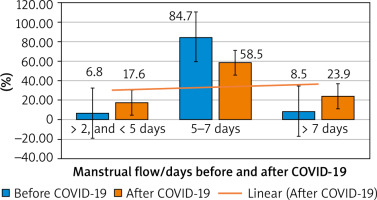
The number of participants who reported menstrual flow for 2 to < 5 days, and menstrual flow > 7 days after the COVID-19 infection [31/176 (17.6%) and 42/176 (23.9%), respectively] was significantly higher compared to the number of participants who reported menstrual flow for 2 to < 5 days, and menstrual flow > 7 days before the COVID-19 infection [12/176 (6.8%) and 15/176 (8.5%), respectively], (p = 0.005 and 0.0009, respectively) (Fig. 1, Table 4).
Table 4
Menstrual flow days and number of pads used/days before and after COVID-19 infection
Moreover, the number of participants who reported normal menstrual flow days (5–7 days) after the COVID-19 infection [103/176 (58.5%)] was significantly lower compared to the number of the participants who reported normal menstrual flow days before the COVID-19 infection [149/176 (84.7%)] (p = 0.02) (Table 4).
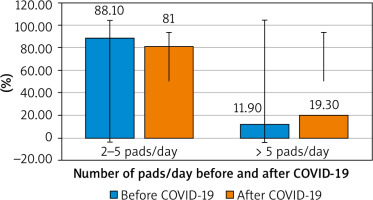
The number of pads used/day did not show any significant difference before or after the COVID-19 infection (Fig. 2, Table 4).
Menstrual changes after the thrombo-prophylaxis or anticoagulants used during the COVID-19 infection
The number of participants who reported increased menstrual flow, menstrual flow for 2 to < 5 days, and menstrual flow > 7 days after the thromboprophylaxis or anticoagulants during the COVID-19 infection [45/119 (37.8%), 22/119 (18.5%), and 71/119 (59.7%), respectively] was significantly higher compared to those who did not use thromboprophylaxis or anticoagulants during the COVID-19 infection [15/57 (26.3%), 9/57 (15.8%), and 32/57 (56.1%), respectively], (p = 0.0002, 0.02, and 0.0007, respectively).
In addition, the number of participants who reported contact bleeding, and abnormal menstrual pattern for one cycle after the thromboprophylaxis or anticoagulants during the COVID-19 infection [7/119 (5.9%) and 8/119 (6.7%), respectively] was significantly higher compared to those who did not use thromboprophylaxis or anticoagulants during the COVID-19 infection [1/57 (1.8%) and 0/57 (0%), respectively], (p = 0.2 and 0.0009, respectively) (Table 5).
Table 5
The studied participants’ menstrual pattern after COVID-19 infection with or without use of thromboprophylaxis or anticoagulants
Discussion
A total of 176 women infected with COVID-19 were included in this questionnaire-based retrospective study, which was conducted at ASMH to detect menstrual changes after the use of thromboprophylaxis or anticoagulants during the COVID-19 infection.
External stimuli, including infections, medication, and organ dysfunctions, can easily disrupt the typical menstrual rhythm [18]. Previous studies reported menstrual abnormalities among women infected with viral infections, including hepatitis B virus (HBV), and hepatitis B virus (HCV) [19], as well as human immunodeficiency virus (HIV) [20].
Menstrual changes during the COVID-19 infection
The number of participants who reported menstrual flow for 2 to < 5 days, and menstrual flow > 7 days after the COVID-19 infection [31/176 (17.6%) and 42/176 (23.9%), respectively] was significantly higher compared to the number of participants who reported menstrual flow for 2 to < 5 days, and menstrual flow > 7 days before the COVID-19 infection [12/176 (6.8%) and 15/176 (8.5%), respectively], (p = 0.005 and 0.0009, respectively).
A retrospective cohort study including 18.076 women and examining the ovulatory and menstrual changes during the COVID-19 pandemic found that 7.7% and 19.5% of the studied participants had anovulation and abnormal menstrual cycle length, respectively, during the COVID-19 pandemic [21].
Another recent retrospective study, conducted to evaluate the menstrual changes after COVID-19 infection, found that 56.9% of the studied participants reported a change of their menstrual blood loss, and 47.2% of them reported a change of their menstrual blood loss and the number of days between 2 consecutive cycles [16].
A retrospective, cross-sectional study including 237 reproductive-age women infected with COVID-19 found that 25% of the studied women had menstrual volume changes, and 28% of them had menstrual cycle changes (mainly decreased menstrual volume or prolonged cycles) [22].
The menstrual abnormalities were also reported after receiving mRNA or adenovirus vector COVID-19 vaccines, which suggest a relationship between the menstrual changes, and the host immune response rather than the virus or the vaccine components [23].
Menstrual changes after the thrombo-prophylaxis or anticoagulants used during the COVID-19 infection
The number of participants who reported increased menstrual flow, menstrual flow for 2 to < 5 days, and menstrual flow > 7 days after the thromboprophylaxis or anticoagulants during the COVID-19 infection [45/119 (37.8%), 22/119 (18.5%), and 71/119 (59.7%), respectively] was significantly higher compared to those who did not use thromboprophylaxis or anticoagulants during the COVID-19 infection [15/57 (26.3%), 9/57 (15.8%), and 32/57 (56.1%), respectively], (p = 0.0002, 0.02, and 0.0007, respectively).
In addition, the number of participants who reported contact bleeding, and abnormal menstrual pattern for one cycle after the thromboprophylaxis or anticoagulants during the COVID-19 infection [7/119 (5.9%) and 8/119 (6.7%), respectively] was significantly higher compared to those who did not use thromboprophylaxis or anticoagulants during the COVID-19 infection [1/57 (1.8%) and 0/57 (0%), respectively] (p = 0.2 and 0.0009, respectively).
The use of anticoagulants was listed as one of the causes of AUB in the PALM and COEIN classification of AUB [15], and it is one of the common causes of AUB [24]. The usage of thromboprophylaxis or anticoagulants in COVID-19-infected women remains an area of debate, and there are no available guidelines regulating the use of thromboprophylaxis or anticoagulants during COVID-19 infection [25].
This study was the first questionnaire-based retrospective study conducted at ASMH, including 176 women with COVID-19 to detect the menstrual changes after the use of thromboprophylaxis or anticoagulants during the COVID-19 infection.
Significant menstrual changes were observed in this study after COVID-19 infection (17.6% of the participants reported menstrual flow for 2 to < 5 days, and 23.9% reported menstrual flow > 7 days). The use of thromboprophylaxis or anticoagulants during COVID-19 was associated with significant menstrual changes (37.8% increased menstrual flow, 18.5% menstrual flow for 2 to < 5 days, 59.7% menstrual flow > 7 days, 5.9% contact bleeding, and 6.7% abnormal menstrual pattern for one cycle). The retrospective nature and the self-reported data were the limitations of the current study.
The menstrual changes after the COVID-19 infection and after the use of thromboprophylaxis or anticoagulants during the COVID-19 infection need to be confirmed in future studies. A guideline regulating the use of thromboprophylaxis or anticoagulants during the COVID-19 should be created and implemented.
Conclusions
Significant menstrual changes after COVID-19 infection were observed in this study (17.6% reported menstrual flow for 2 to < 5 days, and 23.9% reported menstrual flow > 7 days). The use of thromboprophylaxis or anticoagulants during COVID-19 was associated with significant menstrual changes (37.8% increased menstrual flow, 18.5% menstrual flow for 2 to < 5 days, 59.7% menstrual flow > 7 days, 5.9% contact bleeding, and 6.7% abnormal menstrual pattern for one cycle).