Introduction
Surgical site infections (SSI) occur at the site where surgery is performed in the post-operative period and account for an estimated 22% of all healthcare-associated infections [1]. The global prevalence of SSIs is 0.5–15% with a higher prevalence of up to 38% in developing and low-middle-income countries such as India [2]. The Centers for Disease Control (CDC) have categorized SSIs depending upon the extent of penetration into the skin or tissues as superficial SSI (involving only the skin or subcutaneous tissue), deep incisional SSI (involving deep soft tissues of an incision), and infections involving organs or body spaces [3]. SSIs are associated with increased morbidity, extended hospitalization stays, and mortality in addition to a significant financial burden on patients and payers due to extended hospitalization stays, treatment costs, and in some cases reoperations [4].
Methods to minimize the occurrence of SSI include the use of infection control measures such as antimicrobial prophylaxis, operating room ventilation, sterilization methods, barriers and drapes, surgical techniques, maintenance of patient homeostasis, and surveillance programmes by surgeons [4]. These strategies can be used during the preoperative, intraoperative, or postoperative phases depending upon the surgery type and surgeon preference and have been recommended by the World Health Organization (WHO) at differing recommendation levels based on the strength of gathered evidence [5].
Caesarean section (C-section) procedures are becoming increasingly prevalent with a global incidence of 1 in 5 childbirths as of 2021 with a projected increase to almost a third of all births by 2030 according to WHO estimates [6]. Complications accompanying C-section births include maternal infectious morbidity, with SSIs being most prevalent as compared to vaginal deliveries [6]. The reported incidence of SSI following C-section ranges from 3% to 15%, causing a physical and emotional burden on the mother as well as significant healthcare-associated financial implications [7]. A prospective observational study conducted in north India reported an SSI rate of 10.3 per 100 procedures, with superficial SSIs (66.7%) being the most common sub-type [8]. A three-fold increase in cost associated with post-caesarean SSI compared to a non-infected matched control group (p < 0.0001) was observed in a study conducted at a tertiary hospital in India indicative of a substantial medical and financial burden on the healthcare system [9]. Risk factors implicated in the development of post-C-section SSIs include patient-related co-morbidities such as hypertension, diabetes mellitus, obesity, parity, prolonged labour, premature rupture of membranes, and emergency C-sections [10–13]. Since C-sections are indicated in several cases for the safety of the mother and child, it is essential to develop measures to prevent complications associated with these procedures that can jeopardize maternal health.
Among the various strategies that the WHO recommended in its SSI prevention guidelines in 2018, negative pressure wound therapy (NPWT) was recommended for use in adult patients on closed surgical incisions in high-risk cases [5]. NPWT involves the application of negative pressure via a closed system to the wound site via tubing that draws fluid away from the incision site. It helps to reduce oedema, promote granulation tissue formation, and accelerate wound healing [14–16]. Closed incision negative pressure therapy (ciNPT) is expected to reduce the incidence of SSIs by protecting the incision from external wound contamination, strengthening the cohesiveness of the wound edges, removing fluids and infectious materials from the wound, decreasing lateral tension around the incision, and allowing for oxygen saturation, lymphatic flow, and blood microcirculation within the incision area [17].
Previous meta-analyses have shown a significant decrease in SSI incidence in women undergoing C-sections who received prophylactic NPWT compared to standard dressings [18–20]. A Cochrane group meta-analysis compared NPWT to standard dressings across various surgery types and showed a non-significant decrease in SSI in orthopaedic (hip/knee arthroplasties and limb fractures), abdominal, and hepatopancreaticobiliary surgery, whereas a significant reduction in SSI incidence was reported in obstetric (C-section), vascular (peripheral bypass), and cardiac procedures [21].
Aim
Based on the promising results of NPWT in C-section procedures, the objective of this paper was to conduct an updated meta-analysis on randomized controlled trials (RCTs) comparing SSI incidence and wound complications in women undergoing C-sections receiving NPWT or standard dressings after wound closure. Furthermore, this study also compares the effectiveness of NPWT interventions by the type of C-section (emergency or elective) and NPWT system used.
Material and methods
The current meta-analysis was carried out in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) initiative’s requirements.
Search strategy
Various databases such as MEDLINE (PubMed), Cochrane Register of Controlled Trials (CENTRAL) and ClinicalTrials.gov were accessed and searched in December 2022 using the following search terms in various combinations: surgical site infection, wound infection, SSI, surgical wound infection, NPWT, and caesarean section. Additionally, a comprehensive list of search terms including Medical Subject Headings (MeSH) terms was applied. The titles and abstracts of studies that were potentially relevant were scanned and the full-text versions of the appropriate articles were read. Additional studies were identified by cross-checking the reference lists of the relevant studies.
Study selection or inclusion/exclusion criteria
RCTs that compared the application of NPWT to surgical incisions with standard wound dressing methods were included in the analysis. All studies reporting SSI or wound infections as outcomes were included irrespective of the definition of SSI used and even if it were not the primary outcome. Other types of study designs were excluded from the analysis due to occurrence of significant biases.
Data extraction and quality assessment
Data were extracted using a predefined data extraction form that included the following items: study author, publication year, participant information, type of C-section (emergency/elective), intervention, control, type of NPWT device, SSI, wound complications, and SSI criteria.
Risk of bias across various domains such as randomization, allocation concealment, blinding, and completeness of follow-up was determined for each included study using the Cochrane Collaboration’s risk of bias tool. The risk of bias for each item was graded as high, low, or unclear risk using the author’s judgement and common assessment criteria.
Quantitative data synthesis
Meta-analysis was performed using Review Manager (RevMan, Version 5. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration. 2020). Absolute numbers of participants in each study developing an SSI and other wound complication (seroma, haematoma, wound dehiscence, wound disruption, abscess, cellulitis) and the total number of participants in each group (intervention and control group) were used to calculate the risk ratio and the 95% confidence interval (CI). Meta-analyses were done using a random-effects model (Mantel-Haenszel method) and heterogeneity in the included studies was evaluated using the I2 statistic, with small heterogeneity for I2 values of 25%, moderate heterogeneity for I2 values of 25% to 50% and high heterogeneity for I2 values > 50%. Forest plots were constructed and p < 0.05 was statistically significant. Subgroup analyses were also performed according to the type of caesarean section (emergency/elective/both).
Publication bias was assessed by a funnel plot in which the log risk ratio for each study was plotted against its standard error for the SSI outcome.
Results
Identification of studies
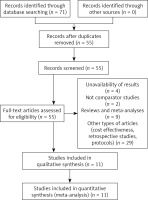
A total of 71 records were identified by database searching, of which 55 were screened by title and abstract. Irrelevant records were removed (n = 44) and 11 RCTs were assessed for eligibility and included in the meta-analysis [22–32]. The process of selection is shown in Figure 1.
Study characteristics
In total, 11 RCTs totalling 5,693 participants met the inclusion criteria (NPWT group: 2844 participants and standard therapy group: 2849 participants). These RCTs involved a comparison of NPWT application vs. standard dressing in participants undergoing caesarean section procedures of various types. All studies were randomized controlled trials, with the number of participants ranging from 54 to 2035. The studies included female participants with BMI ≥ 30 kg/m2 who underwent C-section procedures which were elective, emergency, or mixed surgery types (Table I). Different types of NPWT systems, PICO (single-use NPWT system) or PREVENA (incision management system) were used in the studies (Table II). Surgical site infection (SSI) was defined according to different criteria in the studies (Table III), and different wound complications such as dehiscence, seroma, haematoma, cellulitis, and wound disruptions were also noted and compared between the two groups (Table IV).
Table I
Characteristics of included studies
| Reference | Country | Participant characteristics | Type of caesarean section (CS) (elective/emergency) |
|---|---|---|---|
| Chaboyer 2014 [22] | Australia | BMI ≥ 30 kg/m2 | Elective |
| Gillespie 2021 [23] | Australia | BMI ≥ 30 kg/m2 | Elective and semi-urgent |
| Gunatilake 2017 [24] | USA | BMI ≥ 35 kg/m2 | Elective and emergency |
| Hussamy 2019 [25] | USA | BMI ≥ 40 kg/m2 | Elective and emergency |
| Hyldig 2019 [26] | Denmark | BMI ≥ 30 kg/m2 | Elective and emergency |
| Peterson 2021 [27] | USA | BMI ≥ 40 kg/m2 | Elective and emergency |
| Ruhstaller 2017 [28] | USA | BMI ≥ 30 kg/m2 | Emergency |
| Stitely 2012 [29] | USA | Weight > 199 lbs | NS |
| Tuuli 2017 [30] | USA | BMI ≥ 30 kg/m2 | Both (mostly elective) |
| Tuuli 2020 [31] | USA | BMI ≥ 30 kg/m2 | Both |
| Wihbey 2018 [32] | USA | BMI ≥ 35 kg/m2 | Both |
Table II
Characteristics of interventions in the included studies
Table III
Surgical site infection (SSI) rates and criteria
Table IV
Wound classification rates
Types of surgery and NPWT devices
Studies were conducted in pregnant women undergoing C-section procedures that were scheduled or emergent. The PICO system or PREVENA systems/devices for negative pressure wound management were used in the intervention group in all studies.
Bias assessment
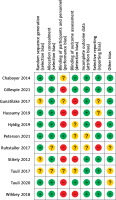
The results of the risk of bias evaluation are shown in Figure 2. Overall, there was a moderate to high risk of bias due to blinding.
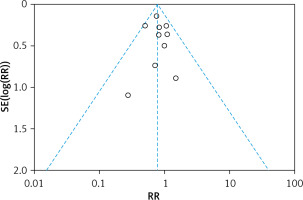
The funnel plot was asymmetrical (Figure 3), indicating the possibility of publication bias.
Surgical site or wound infection rates
The incidence of surgical site infection or wound infection in the included studies is shown in Table III [22–32]. The SSI incidences ranged from 2.6% to 22.7% in the NPWT group and from 3.3% to 27.9% in the standard dressing group. The overall incidence of SSI was 6.5% in the NPWT group and 8.3% in the standard dressing group.
Meta-analysis results
The results of the meta-analysis for all the studies included showed a significant reduction in the incidence of SSIs and wound complications irrespective of the type of C-section (emergency/elective) or the type of NPWT system used (RR = 0.79, 0.66–0.95, p = 0.01, I2 = 0% for SSI incidence and RR = 0.86, 0.75–0.98, p = 0.02, I2 = 5% for wound complications) (Figures 4 and 5). Low heterogeneity could be attributed to the similarity in the type of patients in the different studies (BMI > 30 kg/m2 indicating obesity).
Figure 4
Forest plot of studies included in the meta-analysis (n = 10) using a random effects model with SSI incidence as the outcome. Risk ratios and 95% confidence intervals are shown
NPWT – negative pressure wound therapy, Standard – standard surgical dressing.
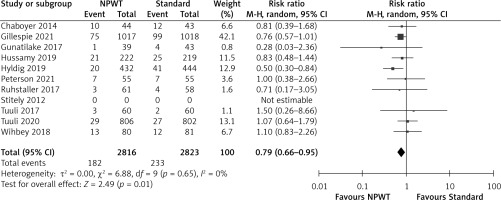
Figure 5
Forest plot of studies included in the meta-analysis (n = 11) using a random effects model with wound complications outcome (seroma, dehiscence, haematoma, bleeding). Risk ratios and 95% confidence intervals are shown
NPWT – negative pressure wound therapy, Standard – standard surgical dressing.
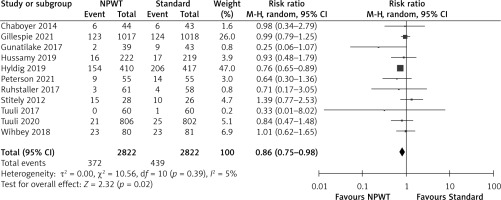
Stratification of the results by the type of caesarean section (emergency/elective/mixed) showed an overall significant decrease in SSI incidence following NPWT treatment over the standard wound dressing (RR = 0.79, 0.66 to 0.95, p = 0.01, I2 = 0%). However, there was no significant difference in SSI rates between the NPWT and standard dressing groups when subdivided by the type of caesarean section with all types showing no significant difference between the NPWT and standard dressing groups (Figure 6). The test for subgroup differences indicated no statistically significant subgroup difference (p = 0.97), indicating that the type of surgery does not influence the response to NPWT. It is of value to note that there was only one study that studied the effect of NPWT in emergency caesarean sections.
Figure 6
Forest plot for subgroup analysis for type of caesarean section in studies using a random effects model on SSI incidence outcome. Risk ratios and 95% confidence intervals are shown
NPWT – negative pressure wound therapy, Standard – standard surgical dressing.
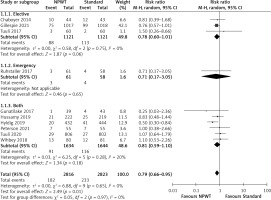
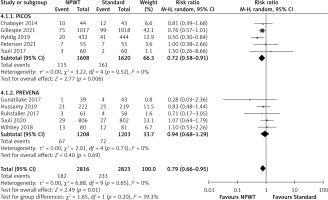
Subgroup analysis was also performed by the type of NPWT system used. Stratification by the device type used showed that the negative pressure system caused a significant decrease in SSI outcome when the PICO system was used (RR = 0.72, 0.58–0.91, p = 0.006, I2 = 0%), whereas there was no significant difference in SSI incidence when the PREVENA system was used versus standard wound dressing (RR = 0.94, 0.68–1.29, p = 0.69, I2 = 0%) (Figure 7). However, there was no significant subgroup effect (p = 0.20).
Discussion
The present study provides current information on the efficacy of NPWT systems in reducing the incidence of SSIs and wound complications in women undergoing C-section procedures.
In accordance with some previous meta-analyses, the current meta-analysis shows the beneficial effect of NPWT in reducing the incidence of SSI when applied to post-surgical incisions and used prophylactically in obese women undergoing C-sections. However, NPWT also seen to have a favourable effect on other wound complications such as dehiscence, haematoma, seroma, and disruption. This paper also studies the effect of NPWT on SSI incidence depending upon the type of C-section (elective/emergency) and the NPWT system or device used and includes all RCTs published until December 2022. In contrast with other studies that have found no significant effect of NPWT in reducing wound complication incidence, this study showed a statistically significant reduction in wound complications (other than SSIs) following NPWT versus standard dressings. However, the clinical significance of this benefit is not certain. Other meta-analyses of interventions to reduce the incidence of SSIs showed support for the use of intraoperative povidone-iodine in different forms such as spray, irrigation, lavage, or scrub in terms of SSI outcomes with a significant reduction of SSI in patients undergoing gynaecological procedures such as C-sections (RR = 0.81, 0.69–0.95, p = 0.009, I2 = 0%) [33, 34].
Since the studies included in the meta-analysis are relatively uniform as regards patient demographics (age, weight, BMI) and clinical characteristics such as co-morbidities, they can be pooled together, and the heterogeneity is low. Despite this, a random-effects model was used in the meta-analysis to account for possible heterogeneities between the studies and, as a result, give more conservative results. The current study only includes data from RCTs which provide the highest level of evidence, which helps to reduce several sources of bias that can occur in comparative studies. Furthermore, a comprehensive search strategy using various databases of published papers and ongoing clinical trials helps to ensure the most current information to help in decision-making.
Although a significant effect of NPWT in reducing SSI and wound complication incidence was detected this study, it is important to note that most individual RCTs included in the analysis showed no significant effect of NPWT as the confidence limits cross the line of no effect (RR = 1). The overall beneficial effect was mainly driven by two RCTs, namely, Gillespie et al. 2022 [23] and Hyldig et al. 2019 [26], where the sample size was large and accounted for nearly half of the total number of participants in the analysis. When the effect of NPWT was analysed according to the type of surgery, a non-significant benefit was observed for elective or mixed (emergency and elective surgery subtypes) despite there being a significant benefit in terms of SSI reduction when all types of surgery were considered together.
It is important to note that although NPWT helped reduce the SSI incidence in C-sections, its benefit does not necessarily extend to all procedures. A pilot study on stoma reversal as part of colorectal surgery showed that closed-incision NPWT did not reduce the incidence of SSI compared to standard sterile dressings (p = 0.651), indicating that this type of procedure may be suitable for only some surgery sub-types and should be applied accordingly [34]. Subgroup analysis revealed that the PICO system showed a significant benefit whereas the PREVENA system did not significantly reduce SSI incidence across all types of C-sections when compared to standard dressings. The differential effects of these devices could be due to better handling and operation of the PICO system by surgical staff or other confounding factors such as antibiotic prophylaxis between the groups, or the duration of application of the devices.
Despite the inclusion of only RCTs in this meta-analysis as well as enough participants for analysis, this study suffers from some limitations. It is important to note that the definition of SSI varied amongst the studies, with the CDC criteria being used in some cases and patient reports and wound scoring systems used in other studies. This non-uniform definition of SSI could have potentially led to erroneous or misleading results. The follow-up time for the occurrence of SSIs ranged from a few days to almost 6 weeks, which also affects the occurrence of SSI and consequently the results of the meta-analysis. Although the current study analysed the effects of NPWT compared to standard dressings on wound complications, the definition of wound complication varied among the studies. Sufficient data were not available for any single wound complication, such as haematoma, dehiscence, or seroma, to be able to give reliable results and thus all were pooled together for analysis.
Other notable factors that differed between the RCTs used in the meta-analysis which have an implication of SSI and wound infections are the type and duration of antibiotic prophylaxis, type of skin closure technique, and the comparator used. In most studies, the type of standard dressing was not specified, which could affect the SSI incidence in the comparator group and lead to varying results. One limitation of this study is the applicability of these results to developing countries such as India, as most of the RCTs were from higher-income countries, mainly the United States. Surgical protocols pertaining to infection control are expected to be much more stringent in these countries compared to developing nations, where it is expected that the incidence of SSI would be much higher. Therefore, it is necessary to have more RCTs performed in developing countries to accurately determine the benefit of NPWT from a global perspective. Regarding the design of the RCTs, the risk of bias assessment showed a relatively high risk of performance and outcome assessment bias. Although double blinding is unavoidable for these studies, it is important to keep this risk in mind when interpreting and extending these results in favour of NPWT. The participants in the RCTs were all obese women with a BMI ≥ 30 kg/m2, in whom SSI risk is higher. It is important to determine whether the beneficial effect of NPWT also extends to cases in which women have a normal BMI or other comorbidities such as diabetes, which has also been associated with an increased SSI risk.
Conclusions
Despite possible limitations, the present meta-analysis indicates that NPWT is associated with a decrease in the risk of SSI in obese women undergoing C-sections regardless of the indication of the surgery (emergency/elective) or the device used. The inclusion of more RCTs with uniform definitions of SSI, NPWT device application times, follow-up time, and comparator dressings is expected to produce more robust results to support the use of NPWT and recommend their use despite their higher costs. Furthermore, to allow for widespread use for the prevention of SSI, it is necessary to include an economic evaluation to justify the cost increase associated with NPWT devices to offset the costs of treatment of SSIs using antibiotics, extended hospitalization stays, or reoperation costs.