Whenever reasonably feasible, strokes should be prevented rather than experienced by stroke victims and their families [1, 2]. Despite the progress in medical therapies, atherosclerotic carotid artery stenosis remains an important, mechanistic risk factor of embolic and hemodynamic stroke even in patients on optimized medical therapy [1, 3]. Emerging evidence indicates that with optimized intraprocedural cerebral protection [4] and routine use of sustained embolic prevention stents [5, 6], today’s endovascular (i.e., minimally-invasive) carotid revascularization (carotid artery stenting – CAS) may be, overall, at least as safe and effective as surgery [7]. Although some specific lesion subsets, such as those highly-calcific or thrombus-containing, have been conventionally more challenging for percutaneous carotid or coronary interventions than for surgery, technological progress increasingly facilitates endovascular management of high-risk lesions [4, 6, 8–10]. This is practically relevant not only for elective CAS (where, to some, surgery remains an alternative) but first of all for emergency CAS because in carotid-related strokes (including tandem strokes) the carotid lesion is mostly highly-thrombotic while in some patients it is also highly-cacific [11].
The decision to perform or defer elective carotid revascularization should ideally be based on a multi-disciplinary (Neuro-Vascular Team) consensus statement [3]. To assist the patient in their decision, the Neuro-Vascular Team may also advise a preferred revascularization mode (according to patient-specific factors and local expertise) [3]. The patient, holding a central position in the decision process regarding their care, requires full information about disease-related stroke risk and treatment options [3].
We would like to share our use of a MicroNET-covered stent-in-stent implantation (‘sandwich’ technique, Figure 1) to seal carotid artery perforation, a rare complication of CAS [12], which occurred during treatment of a highly-calcific carotid lesion in an acute carotid syndrome patient who was rejected from surgery.
Figure 1
Procedural images demonstrating the MicroNET-covered stent ‘sandwich’ technique to seal calcific carotid artery perforation. A is a baseline cerebral angiogram in a 64-year-old male diabetic patient with left internal carotid artery (LICA) chronic total occlusion (CTO; past left-hemispheric stroke, mRS 2) and a symptomatic highly-calcific carotid stenosis on the right (recurrent transient ischemic attacks), cleared by the Neuro-Vascular Team for endovascular management rather than surgery due to severe heart failure, neurologic consequences of prior left hemispheric stroke, and multiple co-morbidities; B shows the right common carotid artery (RCCA) distal non-obstructive diffuse lumen defects and a tandem (yellow arrowheads) stenosis of the right internal carotid artery (RICA) with critical severity in the proximal portion (note projecting calcific nodules); C is a zoomed non-contrast image of the treatment target zone; note massive calcifications forming a calcific “cast” of the index carotid bifurcation (Grade 4 severity of calcifications; circularity index 4 [9]), extending to the proximal right external carotid artery and involving a long segment of RICA (cf., E); D predilatation of the proximal (tight stenosis) portion of the tandem lesion (preceded by placement of a neuroprotective filter in distal RICA – Spider FX 7.0 mm, white arrowhead); initially 3.0 × 12 mm non-compliant (NC) balloon was used, followed by 4.0 × 15 mm NC balloon and 4.5 × 15 mm NC balloon (step-wise carotid calcific lesion preparation as per the PARADIGM Study HCCS Management Protocol [9, 11]); E shows positioning of a MicroNET-covered neuroprotective self-expandable stent (CGuard 9.0 × 40 mm); note that the stent insertion through the distal portion of the tandem required predilating also of the angiographically non-critical distal stenosis; yellow arrowheads denote angiographically-evident calcifications; F is a photograph of the MicroNET-covered stent, consisting of a widely open-cell (area of ~22 mm2, ensuring high adaptability) laser-cut nitinol frame that is wrapped into an embolic prevention MicroNET sleeve (microcell size ~0.02–0.03 mm2) positioned outside the stent frame [18, 19, 21, 22]; G shows post-dilatation optimization of the proximal portion of stented lumen, using a 5.5 × 15 mm NC balloon (arrows denote balloon markers); this step was preceded by sequential embedment of the stent with a 5.0 × 20 mm NC balloon; H is a dye injection demonstrating a focal perforation (red arrowheads) in the mid portion of the stent, causing contrast extravasation; I shows a sealing attempt by performing low-pressure balloon inflations (arrows denote balloon markers); J demonstrates lack of efficacy, in this case, of balloon sealing as the first-line management (red arrowheads point to continued contrast extravasation); note that this angiographic evidence of perforation was accompanied by gradual formation of a neck hematoma; K is implantation of the 2nd MicroNET-covered stent (stentin-stent ‘sandwich’ technique; CGuard 8.0 × 30 mm, white arrowheads) to increase the sealing density effect; this was followed by a gentle balloon inflation to optimize the apposition of the sealing layers; L is a control angiogram demonstrating a reduction – but not yet cessation – of contrast extravasation (red arrowheads); M shows implantation of the 3rd MicroNET-covered stent (CGuard 8.0x30 mm; stent-in-stent-in-stent, white arrowheads) to further maximize the perforation sealing; N is the final balloon inflation for optimized embedment of the 3 (stent + MicroNET) layers; O shows an optimal final angiographic result at the target lesion level of a stenosis-free reconstruction of the target vessel supplying both the right and left cerebral hemisphere; P is the final end-organ angiogram, demonstrating improved cerebral perfusion (white arrowheads, cf., A) in absence of any procedure-related neurologic symptoms
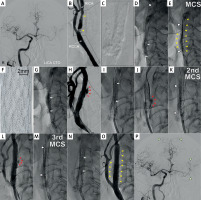
Carotid artery perforation is a rare but serious complication [12, 13]. Emergency management of severe leaks includes external compression (if anatomically feasible) and either surgery (reconstruction or ligation [13]) or endovascular treatment. Conventional endovascular options include carotid artery sacrifice (coiling) to stop bleeding (while risking severe neurologic complications) or perforation sealing with the use of a covered stent (stent-graft) [14, 15]. More recently, effective use of MicroNET-covered stent has been reported in sealing carotid [13] or coronary perforations [16, 17]. The MicroNET-covered carotid stent consists of a widely open-cell (area of ~22 mm2, ensuring high adaptability) laser-cut nitinol frame that is wrapped into embolic prevention [18–20]. MicroNET sleeve (microcell size ~0.02–0.03 mm2) positioned outside the stent frame [18, 19, 21, 22].
In minimal-to-moderate leaks, the initial strategy may involve a sealing attempt with prolonged balloon inflations [23]. Prolonged balloon inflations, however, may increase the risk of symptomatic thrombosis, particularly if performed in absence of procedural anticoagulation or with premature (i.e., prior to removal of the interventional devices from the vessel lumen) heparin reversal with protamine [23]. In our patient, protamine reversal of heparin was performed immediately after removal of the filter and guiding catheter from the arterial system.
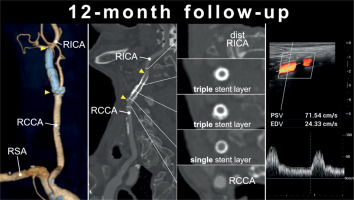
In contrast to the previously reported [13] effective sealing of perforation in a non-diseased carotid artery with a single MicroNET-covered stent, sealing of the highly-calcific internal carotid artery required creating a layer of 3 MicroNET-covered stents; a new interventional solution (Figure 1). 12-month clinical follow-up was normal, and computed tomography angiography and Duplex ultrasound showed an optimal anatomic and functional effect and, despite the 3 stent + MicroNET layers, with luminal flow normal for a single MicroNET-covered stent and absence of any in-stent restenosis (Figure 2). This is consistent with the MicroNET-covered stent normal healing [22, 24–26], and a lasting effect of optimal endovascular reconstruction of carotid artery perforation using a MicroNET-covered stent-in-stent technique, in agreement with a lasting mid-term and long-term effect of MicroNET-covered stent use to restore normal carotid artery lumen in elective stroke prevention and in emergency treatment of carotid-related stroke [11, 22, 24–26].
Figure 2
Computed tomography angiography and duplex ultrasound follow-up of CGuard stent-in-stent-instent implantation (3 MicroNET-covered stent layers) to seal perforation a highly-calcific carotid artery. A is computed tomography angiography 3D reconstruction demonstrating, 12 months post-procedure, a full reconstitution of normal anatomy with a normal contrast opacification of the right internal distal carotid artery (RICA) distal to the stent layers; arrowheads mark edges of the outer stent (CGuard 9.0 × 40 mm); RCCA – right common carotid artery, RSA – right subclavian artery; B is a longitudinal section through the RCCA and RICA, demonstrating a normal lumen inside the 3 MicroNET-covered stent layers in RICA and the stent(s) blooming artifact (note also calcium outside the stent(s) layers); the stented segment marked with arrowheads; cf., 1 B–E); C are cross-sectional sequential images of stented RICA/RCCA segment including the distal RICA reference (top; dotted), triple-stent layers, and proximally a single stent layer and the proximal reference (RCCA; dotted); note a more pronounced blooming artifact from the triple than that from a single stent layer; D are control duplex ultrasound images at 12 months, demonstrating an optimal long-term lumen reconstruction with absence of any intraluminal material (top) and a normal flow spectrum (and velocities) as seen in a typical follow-up of single-layer MicroNET-covered stents (cf., Refs [24–26])
Besides traumatic injury [13], carotid artery perforation can occur as a complication of carotid artery stenting [12] particularly in case of endovascular management of highly-calcific lesions using the 1st generation (single-layer, non-covered) carotid stents [26]. Intraluminal angioplasty has the potential to cause tearing or rupture of the target vessel; a likely under-reported serious complication. A single-layer nitinol stent is believed, in general, to reinforce the wall of the artery [23]. With angioplasty, the force is transmitted to the circumference of the vessel wall, with only the weakest portion failing [23]. Speculatively, such angioplasty-inflicted tears that may be reinforced by stent placement [23]. Nevertheless, in case of heavy calcification any “aggressive” optimization of a conventional (non-covered) stent may cause perforation [27]. On the other hand, calcification is the most challeging cause for post-procedural residual stenosis – a fundamental factor of in-stent restenosis and stent thrombosis [28–30]. Therefore, heavy calcifications have been considered a contraindication to endovascular revascularization using single metallic layer (non-covered) stents [27]. While the MicroNET stent wrap was originally developed to block intraluminal migration of the atherothrombotic plaque material [10, 18, 19, 22], and it was subsequently demonstrated to be effective in this indication [5–7, 20–22], recent analysis from the PARADIGM-101 study suggested efficacy of microNET-covered (CGuard) stent use in optimized endovascular management of highly-calcific carotid artery stenoses [9]. However, it needs to be remembered that the microNET-covered stent is not a stent-graft as it possesses micropores of 150–180 μm [18, 19, 21, 22].
Coiling, an endovascular equivalent of surgical ligation, is associated with a greater stroke risk than that seen with reconstructive techniques that maintain vessel patency and thus ipsilateral cerebral supply [13]. Target artery sacrifice was not considered an option in our patient with a chronic occlusion of the contralateral carotid artery (Figure 1).
Covered stents (stent grafts) were demonstrated to be useful as an effective emergency tool to seal severe perforations (or lesser perforations that persist despite prolonged balloon inflation(s) [23]. However, the risk of in-stent restenosis with fully covered stents (stent grafts) positioned in the carotid location is as high as 15–40% [15, 31], smilar to the restenosis risk occurring with covered stent use in coronary bypass angioplasty [15]. Thus, there is a need for an alternative to exposing patients to such a high restenosis rate. The MicroNET-covered stent, by design, respects carotid anatomy [19, 21, 22], and – when properly embedded – shows a minimal risk of restenosis [5, 22, 24–26]. In contrast, covered stents show lack of mechanical flexibility [15, 31] and a prohibitively high (at least in elective use) rate of in-stent restenosis [15]. Furthermore, contrary to typical expectations [28], covered stents fail to prevent lesion-related thrombo-embolism as the embolic material (rather than getting trapped between the stent and the vessel wall) may get squeezed-out and mobilized to blood stream at the covered stent edges [32].
We report effective and uncomplicated sealing of iatrogenic carotid artery perforation using a MicroNET-covered stent ‘sandwich’ technique (Figure 1) with a lasting, optimal angiographic and clinical result (Figure 2). This result is consistent with accumulating evidence for optimal short- and long-term outcomes with the MicroNET-covered stent system in treatment of carotid artery thrombo-atherosclerotic disease in stroke prevention and in acute stroke [5, 11, 20–22, 25]. Still, operators should be fully aware that the MicroNET-covered stent system is not fully-covered (i.e., it is not a stent graft). Thus, in case of potential incomplete sealing of the leak with a single MicroNET-covered stent [13] or using the MicroNET-covered stent ‘sandwich’ technique (Figure 1), stent grafts [31] should continue to be available on-shelf for major ruptures that may not be amenable to sealing with the micro-porous MicroNET-covered stent(s). While use of scoring balloons and cutting balloons for calcium rupture more ‘controlled’ than that occurring with non-compliant balloons [8] may increase the safety of highly-calcific carotid lesion CAS [11], use of ultrasonic pulse waves to micro-fracture calcium deposits in the vessel wall (thus turning a non-compliant lesion into a compliant one) is particularly promising [8, 33].
In conclusion, we have demonstrated a safe and effective use of a MicroNET-covered stent system ‘sandwich’ technique to resolve iatrogenic carotid artery perforation of a highly calcific stenosis (Figure 1) in absence of any in-stent restenosis (Figure 2).








