Introduction
Thoracic aortic aneurysms are among the most frequently diagnosed diseases of the circulatory system [1]. They remain asymptomatic for a long time. This is especially true for aneurysms of the thoracic aorta. Symptoms begin to occur when the aorta is significantly enlarged and compression syndrome develops. In the case of the thoracic aorta it may be a feeling of pressure behind the sternum, dyspnea at rest or hoarseness [2]. The clinical classification should distinguish between chronic diseases and acute aortic syndromes, which constitute a completely separate chapter in the diagnosis and urgency of indications for surgery. Among the many causes of aneurysmal aortic dilatation, degenerative changes in the aortic wall or defects in connective tissue, such as Marfan, Ehlers-Danlos or Loeys-Dietz syndromes, should be taken into consideration [2].
In some cases of aortic aneurysms, the root is dilated at the level of the Valsalva sinuses. This pathology is very often accompanied by a valve defect in the form of significant valve insufficiency – resulting from the functional dilatation of the aortic annulus. Less commonly, the valve degenerates. For a long time, this type of malformation necessitated the replacement of the root and valve as described in 1968 by Hugh Bentall and Anthony de Bono [3]. Currently, in patients with significant regurgitation but otherwise healthy aortic leaflets, aortic valve reimplantation (VSRR – valve sparing root replacement) is the surgery of choice.
For the first time, an operation of this type was performed and described by Tirone David in 1995. The procedure was performed by total sternotomy [4]. This technique has undergone several important modifications in recent years leading, among other advances, to the minimization of accesses. The most important part of the root reimplantation procedure is to create a new aortic root and reimplant the patient’s own valve onto it. The neo-root is created with the Dacron vascular graft.
Perioperative imaging
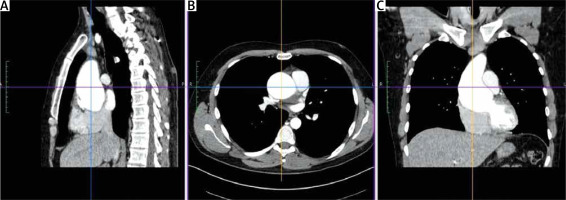
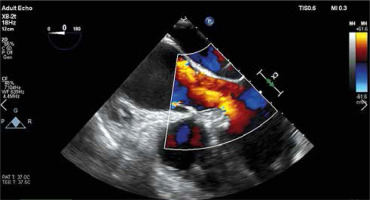
The key element of proper preparation of the patient for the surgery is the performance of chest computed tomography (CT) with 3D reconstruction to assess the ratio of the aneurysm to the sternum surface and the type of aortic position (right, central, left). Such assessment facilitates and streamlines the decision process about the skin incision site and the transverse cut of the sternum (Figure 1). Transesophageal echocardiography remains the most important functional examination in the assessment of aortic valve insufficiency and is essential in the intraoperative assessment of the competence of the performed reimplantation (Figure 2).
Preparation
The patient is under general anesthesia and intubated endotracheally. A central catheter is inserted into the internal jugular vein and pressure is measured invasively from the radial artery. A urinary catheter with temperature measurement is inserted into the bladder. Defibrillation pads are located on the back. The patient is placed on the back with the hands along the body. In the next stage, the chest, abdomen, groin and lower limbs are washed with an antiseptic liquid. The patient is covered with a sterile drape.
V-shape ministernotomy
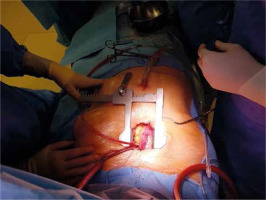
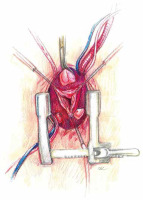
A skin incision is made over the handle of the sternum up to the 3rd or 4th intercostal space. The skin incision is usually about 5–6 cm. Its length depends on the structure of the chest and the length of the sternum. Subcutaneous tissue up to the fascia is dissected using cautery. At the level of the 3rd or 4th intercostal space on both sides, the fascia is completely dissected just above the upper edge of the sternal attachment of the rib. Then, arrow-shaped lines directed with the tip downwards are drawn from the notch of the zygomatic sternum to the designated two parasternal points. Then the breastbone is cut with an electric saw. In the next stage, thymic fat is dissected from the brachiocephalic vein. The pericardial sac is opened longitudinally and to the sides at the lower end as with a standard pericardial inverse “T” incision. On each side of the sternum, 3–4 pull-back pericardial sutures are placed, which are tied to the subcutaneous tissue in such a way that the pericardial sac covers the cut parts of the sternum. In the next stage, a site for cannulation and the aortic clamp is identified and prepared. The ascending aorta and the brachiocephalic trunk are mobilized (Figure 3).
Central cannulation
Central cannulation is performed as the first choice. Heparin is administered at a dose of 3 mg/kg body weight intravenously titrated to the activated clotting time higher than 450 s. In the area of the brachiocephalic trunk, two Ti-Cron 2-0 purse - string sutures are applied. The aorta is cannulated using an EOPA cannula (Medtronic Inc, Minneapolis, MN, USA) and airlessly connected to the extracorporeal artery line. Venous outflow is provided by insertion of an MC2X two-stage cannula (Medtronic, Inc) into the right atrium. The cannula is then pulled out of the chest under the sternum through a small handrail under the xiphoid process, through which the chest tube is carried out after the surgery (Figure 4). At this stage, it is possible to start extracorporeal circulation. With the emptied heart, a 16 F vent (Medtronic Inc, Minneapolis, MN, USA) is inserted through the right superior pulmonary vein into the left atrium. The patient’s body temperature is maintained at 34°C.
Aortic aneurysm resection. Valve sparing root reimplantation
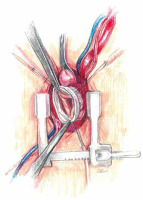
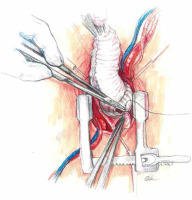
At low flow, a transverse clamp is placed on the border of the distal part of the ascending aorta and the brachiocephalic trunk. The aorta is opened transversely and cold Custodiol (Essential Pharmaceuticals, LLC, Durham, NC) cardioplegia is administered directly to the coronary ostia at a single dose [5]. First, the coronary orifices are excised and the tissue surrounding the initial epicardial course of the coronary arteries is mobilized. Prolene 4-0 mattress sutures are placed on each prepared “button” for gentle traction and improved exposure. After mobilization of the openings, the coronary sinuses are excised, leaving a tissue margin of several millimeters just above the aortic annulus. Ti-Cron 2-0 pledgeted sutures are placed on each prepared commissure (Figure 5) for better exposure. Mosquito forceps are fixed at the ends of the seams providing gentle traction. This simple movement significantly improves visibility in the operating field. At the next stage, the length of the leaflets the height of the commissures are measured and the size of the “neo-root” is selected. The aortic annulus is measured with valve gauges for mechanical prostheses. All measurements taken at this stage are crucial because on their basis the aortic root will be reconstructed. Graft sizing is based on the height of the commissure between the left and the noncoronary sinuses. The height is measured with a ruler from the base of the interleaflet triangle to the top of the commissure, because this measurement corresponds to the size of the graft that will be used. Ethibond 2-0 ring sutures are placed horizontally from the left ventricle aspect to the outside of the aortic annulus. Placing them tightly next to each other, in a tiled shape, helps to stabilize the annulus. After selecting the size of the aortic graft, the sutures are placed successively on the aortic prosthesis and tied to the annulus. In general, straight prostheses are used (Hemashield Platinum Woven Double Velour, Getinge AB, Göteborg, Sweden). In the next stage, the aortic root is trimmed inside the neo-root (Figure 6). Previously performed measurements are used to properly arrange the height of the commissures and leaflets on the wall of the aortic prosthesis. With the Prolene 5-0 suture, the commissures within the margin of the aortic annulus are sewn to the prosthesis. Sewing starts from the nadir of the commissure and goes up towards to the top. In the next step, the competence of the valve and the coaptation of the leaflets are checked in saline testing. If necessary, repair of the valve leaflets is performed at this time. In the aortic prosthesis, at the height of the Valsalva sinuses, round holes for the coronary ostia are excised. The distance to the holes is determined on the temporarily unvented left ventricle to prevent kinking of the coronary vessels when the aortic clamp is removed. The parachute technique uses the Prolene 5-0 suture to sew the left and then the right coronary ostia (Figure 7). In the last stage, the trimmed distal part of the prosthesis is fixed to the aortic arch with the Prolene 5-0 suture. The suturing of the anastomosis begins on the posterior wall of the aorta, heading initially towards the pulmonary trunk and then towards the operator. Before the anastomosis is completed, the heart is vented and the aortic clamp is removed. De-airing of the heart is performed using the anti-Trendelenburg position, filling the lungs and heart, and an active vent from the left ventricle. After the distal anastomosis is closed, the patient is heated to normothermia.
Completion
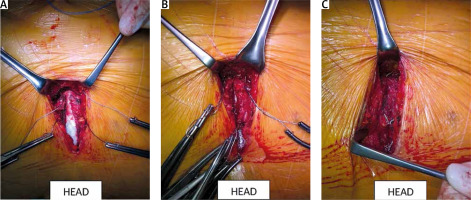
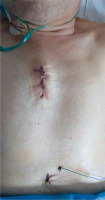
The competence of the aortic valve is checked again by transesophageal echocardiography. Careful and meticulous hemostasis is performed. BioGlue Surgical Adhesive (Jotec, Kennesaw, Georgia, United States) is used to buttress the suture lines. A bipolar electrode is placed for temporary stimulation at the anterior aspect of the RV outflow tract. The patient is weaned from extracorporeal circulation. The cannulas are removed and protamine sulfate is administered in a 1 : 1 ratio. A single 28F chest tube is inserted into the anterior mediastinum and exits under the xiphoid process. The pericardium is closed over the aorta. A gentamicin sponge is used for sternal wound infections prophylaxis. The breastbone is closed with three metal sutures. The subcutaneous tissue and the skin are closed in layers (Figures 8 and 9).
Discussion
Modern cardiac surgery offers a variety of less invasive techniques in the surgical treatment of complex heart and aortic disorders. It has been proven that reducing the invasiveness of the procedure allows for pain reduction and acceleration of postoperative convalescence. The overarching goal of the treatment is to shorten the hospitalization period and to enable the patient’s quick return to society [6–8]. The current paper presents a detailed description of the technique of aortic valve reimplantation as described by David with the difference being access through a V-shaped ministernotomy. Experience in performing partial ministernotomy has been gained thanks to an extensive program of minimally invasive aortic valve surgery. In the first stage, access was performed using a V ministernotomy, then a J-shaped approach, and now, access exchanges are performed using an anterolateral right minithoracotomy. The V-shaped upper ministernotomy, due to the bilateral opening of both cut parts of the sternum, enables good visibility of both the aortic root and the brachiocephalic trunk area. The most important part of this particular operation is good visualization and elevation of the aorta. This is achieved by pulling the pericardial sac up to the level of the chest. Another important issue is properly performed cannulation. Central cannulation is the technique of choice but peripheral cannulation should be at least as safe. An important element is very high cannulation of the aorta, even behind the brachiocephalic trunk. This is possible thanks to the use of flexible cannulas. This type of cannulation leaves a large unobscured space for clamping the aorta. In cases where arch cannulation is not possible, the right subclavian approach is used. Due to the possibility of vascular complications, femoral arterial access is avoided in patients with peripheral vascular disease. Retrograde arterial perfusion is associated with a higher risk of stroke and dissection in the case of minimally invasive mitral valve surgery but may be considered after no atherosclerotic lesions in the aorta were found at CT [9]. An alternative to the reservoir cannula in the right atrium is peripheral femoral vein cannulation, most often using the percutaneous technique [9, 10]. By choice, the vent is inserted through the right superior pulmonary vein and led out together with the venous cannula under the xiphoid process. Due to this solution, there are no unnecessary tools or cannulas within the narrow field. Both the venous cannula and the vent are pulling the right atrium down, and in particular the right atrial appendage, greatly improving the visibility of the aortic root. The main stage of the operation is the sequential reimplantation of the aortic valve and the creation of a new aortic root. The neo-root is made of a vascular graft. The most commonly used grafts are pre-formed Valsalva sinuses [10–13]. Prostheses with embedded sinuses imitate a native aortic bulb. This design facilitates the reimplantation of the leaflets and commissures inside in the prosthesis. The native valve ring is fixed and stabilized by sutures with pledgets, which are inserted from the left ventricular side. Then, commissures and valve leaflets are sewn onto the wall of the prosthesis. In approximately 5% of root aneurysms, a bicuspid aortic valve (BAV) is found, which does not constitute a contraindication for its reimplantation [13]. An equally important element of the operation as the reconstruction of the aortic root geometry is the anastomosis of the coronary vessels in such a way that, after removing the clamp, they do not accidentally bend and the flow in the coronary circulation is impaired. Therefore, cutting openings for the orifices is performed on a partially filled heart. Properly and carefully performed hemostasis is important at each stage of the procedure. After performing the coronary anastomoses, the surgeon is unable to re-inspect the posterior part of the anastomosis and the place where the left coronary button is sewn onto the prosthesis. Biological glue is used to seal the joints along the sutures line. If lengthy aortic root repair is predicted, Custodiol cardioplegic solution is the first choice. In other cases myocardial protection is ensured by cold blood cardioplegia [14]. A very important part of the procedure is the rigid fixation of the sternum with metal sutures. In the described technique, the breastbone is cut in 3 places and must be carefully stabilized with metal wires. In order to prevent infection of the sternum, a gentamicin sponge is applied [15]. The development of medical technologies and equipment used for operations causes that the cuts are becoming more precise and smaller. It has a big impact on the final results of the treatments [16]. Performing David’s operation with V-shape ministernotomy is feasible and safe in a selected group of patients. The experience gained in performing aortic valve replacement using a minimally invasive technique is not without significance. During the surgery via the mini approach, one has to think about how to make this procedure easier. Firstly, central cannulation is important, especially the venous cannula, which is pulled out under the sternum. This maneuver creates more space between the aortic root and right atrium. Secondly, one has to use tension sutures, which are anchored and attached at the level of the coronary buttons and all the commissures. This continuous traction provides good visibility to the leaflets and upper margin of the aortic annulus and simplifies all the measurements. All of the above tips originate from our minimally invasive aortic valve surgery experience. From our experience no contraindications exist to minimally invasive aortic root surgery other than the size of the aneurysm (> 70 mm), chest wall defects, morbid obesity, re-do surgery and congenital heart disease with abnormal origin of the coronary arteries or extra coronary. The beneficial effects are observed in the postoperative course of patients. They primarily comprise accelerating the mobilization of patients, shortening the hospitalization time and reducing the transfusion of blood products [16]. It is not without significance that such operations are performed more and more often in young people who are very active professionally and socially. Keeping one’s own valve protects these patients from the need for life-long anticoagulation. In the case of progressive degradation of the valve, an alternative in the future is the transcatheter implantation of a biological prosthesis into the aortic position: the valve-in-valve procedure [17]. Aortic root operations via V-shaped ministernotomy are safe and can be performed in a selected group of patients [18]. A multicenter registry (Minimally Invasive Aortic Root and Aorta surGery rEgistry) MIRAGE (NCT:04814238) will shed further light on the safety and durability of this approach [5, 19].