Introduction
The liver remains a great challenge for surgeons. It is an organ vastly supplied in blood, receiving a dual blood supply covering circa 25% of resting cardiac output. Due to these facts, massive blood loss was a main concern in liver surgery since its beginning. Therefore, procedures in an elective mode were delayed to the 1950s [1]. Partial hemorrhage control was achieved due to implementation of the Pringle maneuver and “finger fracture” technique, but it is still associated with high morbidity and mortality rates [2]. In the early 1990s the laparoscopic approach, as well as other minimal invasive techniques [3], was applied for liver surgery, starting with wedge resections of small benign tumors. Since then, various new techniques and equipment have been invented for decreasing intraoperative blood loss and transfusion rate, simultaneously with decreasing mortality and morbidity [4].
Aim
The aim of this literature review was to summarize the best available methods for achieving minimal blood loss in laparoscopic liver surgery, according to the newest data.
Imaging in laparoscopic liver surgery
The complex liver anatomy provides several difficulties even to experienced surgeons. With the development of technology, better preoperative and intraoperative visualization of liver anatomical structures and variations, transection planes and tumor relations to the large vessels is a key to success in laparoscopic liver surgery (LLS). Therefore, blood loss control during LLS starts before the surgery, during analysis of preoperative imaging.
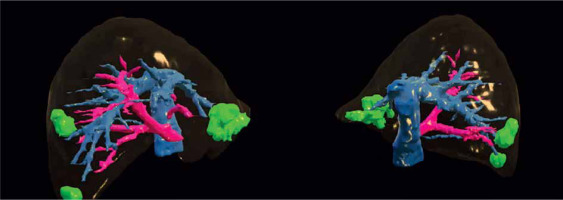
Preoperative imaging is necessary in LLS both for oncological staging and planning the operation with its potentially expected difficulties. The main imaging devices used before LLS are computed tomography (CT) and magnetic resonance imaging (MRI) [5], with CT being more effective in evaluating tumors in relation to large vessels and MRI being more accurate in assessing tumors’ character and evaluating bile ducts [6]. PET-CT imaging is used only in selected cases [7]. 3D printings of liver models are not widely used because of their high cost, but they may impact surgeons’ intraoperative decisions [8]. Moreover, 3D preoperative liver reconstructions, available preoperatively as well as intraoperatively, may be helpful in selected cases (Photo 1) [9]. 3D reconstructions may be helpful in carefully planning difficult liver surgeries including tumor size, location and its relation to large liver vessels [10]. Nevertheless, more prospective studies are needed to possibly introduce this novel technique to everyday practice.
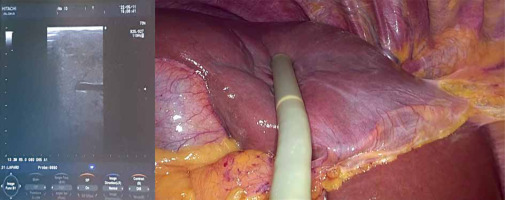
Intraoperative ultrasound (Photo 2), with its low cost and high availability, is a necessary tool for every surgeon performing LLS due to its high sensitivity and ability to moderate the lack of haptic feedback during parenchyma examination [11]. It can assess the precise location of the tumor and its anatomic relations and helps plan intraoperatively transection lines avoiding unexpected collision with large hepatic vessels. Therefore, using intraoperative ultrasound is recommended by numerous authors [12, 13]. Indocyanine green (ICG) fluorescence imaging-guided LLS is a less common technique that uses the mechanism of binding of ICG to plasma proteins excreted only by the liver, but not by the lesion. ICG may be administered preoperatively, most commonly 10 days before the scheduled operation, to better identify the tumor, which preserves the dye, whilst normal liver parenchyma washes it out during that time. ICG can also be given intraoperatively to help identify the intersegmental planes of the liver, which is beneficial in performing anatomical resection, but this technique requires vast experience in LLS [14]. ICG is becoming a more common technique and, according to recent data, may lead to lower blood loss (BL) and postoperative complications. However, more multi-center prospective studies are needed [15].
Image-guided surgery (IGS), such as augmented reality, is another novel technique in LLS. This system merges the preoperative 3D CT scan with the laparoscopic image, providing intraoperative real-time guidance [16], which may significantly help reduce blood loss in LLS in the future. However, due to technical difficulties, such as still insufficient accuracy in liver surface reconstruction, combined with its high cost and a requirement of hybrid operation rooms, augmented reality is not used yet in clinical practice [17].
Liver vascular occlusion
The Pringle maneuver (PM) originated at the beginning of the 20th century, being initially used to control bleeding in emergency cases [18]. its application has evolved over time significantly, making it an inexpensive and efficient bleeding control technique in LLS [19, 20]. Pedicle clamping may result in postoperative transient hypertransaminasemia or hyperlactatemia, but when appropriately performed should not impair short- and long-term results [21]. Initially PM was performed reactively to stop severe bleeding. Better recognition of its potential negative effects has moved towards protective usage of PM to keep the transection plane dry and avoid intraoperative hemorrhage.
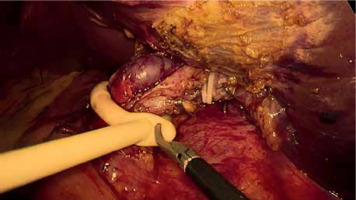
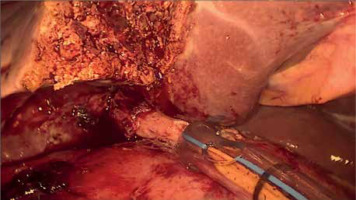
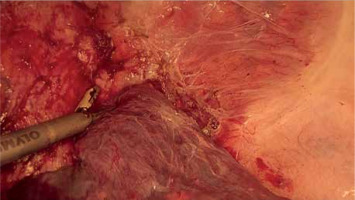
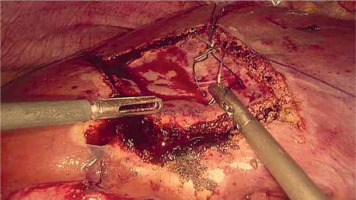
PM should be prepared before parenchymal transection for possible immediate application. It can be performed intracorporeally and extracorporeally. For an intracorporeal PM a long cotton tape is placed around the hepatoduodenal ligament through a 12 mm trocar, then passed through a small tourniquet and clipped 2–3 cm above it. For occluding the hepatic inflow, the tourniquet is pushed toward the hepatic pedicle and clipped above it. Pedicle clamping may also be performed with a laparoscopic bulldog clamp or appropriately prepared Foley’s catheter (Photo 3) [22]. For an extracorporeal PM the tape is externalized through a trocar and passed through the catheter placed above the trocar. The main advantage of the extracorporeal technique is the possibility for clamping by the assistant surgeon, without distraction of the main surgeon (Photo 4). When comparing both techniques of PM in retrospective studies, extracorporeal PM carries more practical advantages, but it still requires further research [23]. PM can be continuous or intermittent (most commonly 15 : 5 min ratio or 10 : 5 min ratio in cirrhotic liver). In HCC patients, according to a recent RCT, 25 min intermittent PM results in lower BL, as well as higher transection speed [24]. However, the performed PM technique depends on surgeons’ preferences and estimated operative time.
Comparing intermittent PM with other vascular inflow occlusion techniques, such as selective vascular occlusion providing local ischemia, can reduce operative time, blood loss and perioperative transfusion rate (TR). However, it can be used only in selected cases and needs careful preoperative planning. Furthermore, due to the paucity of data more prospective studies are needed [25].
To sum up, application of the Pringle maneuver has evolved significantly since its onset and is used widely in LLS, particularly in larger resections. Types and techniques of PM differ depending on surgeons’ preferences and type of procedure. PM is a cheap and available technique for avoiding intraoperative hemorrhage and for better visualization, which is especially important in laparoscopy, but more prospective studies are needed to evaluate its impact on postoperative blood loss and transfusion rate.
Transection method
Based on Couinaud’s classification of liver segments, anatomic liver resection (AR) is the complete excision of at least one of the Couinaud’s segments with the related portal triad branch, whereas non-anatomical resection (NAR) is a local resection regardless of the parenchyma blood supply. When comparing the two techniques, the results are similar in terms of BL, whereas in the NAR group TR was increased [26]. However, the choice of approach depends mostly on tumor location and need for parenchyma sparing [27].
In laparoscopy, there is a different approach to liver segments when compared to open surgery. Due to the various difficulties of the procedure related to the tumor location, with superior posterior segments being overall more difficult to resect than anterior ones, new classifications are proposed such as the Institut Mutualiste Montsouris (IMM) classification, which divides patients into 3 groups based on predicted difficulty of the procedure [28], including 3 main factors: operative time, estimated blood loss and conversion rate risk. Group I represents less demanding cases, such as left lateral resection or wedge resection, more appropriate for surgeons with less experience in LLS. Group II represents an intermediate level in LLS including resection of anterolateral segments, as well as left hepatectomy. Group III, comprising major LLS and resections of superior posterior segments, represents the most difficult cases. Also, estimated blood loss rises in successive groups; therefore implementation of this scale helps to predict which of the hemostatic techniques may be useful in the most difficult cases, to reduce estimated BL. Moreover, IMM is becoming a valuable difficulty scoring system also in laparoscopic repeat liver resection procedures [29]. Another scale that may predict difficulty of the procedure in LLS is the 4-grade IWATE criteria. It is based on tumor location, size, its proximity to large vessels and type of procedure [30].
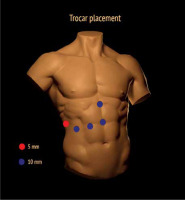
With posterosuperior segment lesions being classified as the most demanding due to the difficulty of achieving them through the abdominal cavity, the thoracoscopic approach may become a viable alternative solution in these cases (Photo 5) [31]. For lesions located in anterior segments, a method called the “diamond technique” enabling optimal use of laparoscopic instruments may be useful, but, due to the paucity of data, its impact on BL and TR is not known yet (Photo 6) [32].
Beside the selected approach, surgeons’ experience is an important factor not only in terms of better instrument handling, decision making and bleeding control, but also due to lower intraoperative BL and operative time, as studies found [33, 34]. According to data, the learning curve of LLS procedures is about 60 cases for minor LLS and then consecutively another 50–55 cases in major LLS after acquiring expertise in minor LLS [35]. This may be shortened by participation in laparoscopic hepatobiliary fellowship programs [36]. Moreover, the difficulty of LLS should be increased gradually according to the IMM/IWATE criteria during surgeons’ learning curve [30]. Another approach in training of hepatobiliary surgeons involves focusing on learning specific skills needed for LLS. A survey conducted by the ILLS (International Laparoscopic Liver Society) identified 22 substeps of LLS, estimating the capacity of a surgical center to provide appropriate training in LLS [37].
Transection devices
Nowadays, during LLS a variety of surgical instruments are used, and the number of them has increased with the development of medical technology. Among them we can highlight devices such as ultrasonic devices, vessel-sealing devices, vascular staples, water jet devices, harmonic scalpels, monopolar and bipolar cautery or more classical methods such as suture ligation [38]. Use of some devices, such as argon beam coagulation, has significantly decreased due to increased risk of gas embolism [39]. Moreover, when comparing the effectiveness of various surgical devices used in LLS, their design to be useful in different clinical situations should be taken into account.
The harmonic scalpel is the main tool used not only in LLS, but in almost every laparoscopic surgical procedure, being precise and providing overall good hemostasis. Harmonic scalpels enable a safe and feasible technique, even in cirrhotic patients [40].
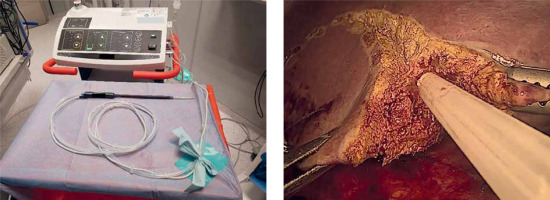
Ultrasonic devices (Photo 7), designed to dissect selectively liver parenchyma utilizing mechanical waves, preserving small vessels and bile ducts and sucking crushed liver parenchyma at the same time, maintain a dry operative field [41]. Therefore, ultrasonic devices are essential in major liver surgery.
Vascular staplers can crush hepatic parenchyma and occlude the hepatic vessels simultaneously. Studies comparing groups with and without vascular staplers show lower blood loss and transfusion rate in the stapler group [42]. However, staplers should be administered carefully and on previously dissected vessels, because blind transection may lead to severe bleeding by vascular injury, especially in deep parenchymal transaction [43].
Bipolar cautery is used in suppressing minor bleeding, being a cost-effective and efficient way to provide intraoperative hemostasis during resection [44].
To conclude, modern laparoscopic liver surgery has a variety of tools that help reducing blood loss and transfusion rate and they serve their purpose best when combined together, showing similar effects on intraoperative blood loss, transection time, morbidity and hospital stay [45].
Topical agents
Hemostatic topical agents (TA) are a group of either biological or synthetic products that are used mainly to maintain intraoperative hemostasis. Three main types of them are collagen, fibrin and cyanoacrylate agents, the third of which are no longer used since they lead to tissue necrosis and release of inflammatory mediators [46]. TA’s mechanism of usage is different – while collagen agents are mainly sheets of collagen-based material that is covered with a hemostatic agent, such as thrombin or protein C or S, fibrin agents are prepared intraoperatively and subsequently applied [38].
Another classification of them is based upon functionality and consists of mechanical, active, flowable and sealant agents. Mechanical hemostats provide a base for rapid clot formation that impedes blood loss, whilst active and flowable agents maintain a local clotting process through several mechanisms, depending on the used material, whereas sealant hemostats work as a tissue glue [47].
With the development of modern surgery, there are plenty of different, often combined TA that are also used in LLS, manufactured by many companies. However, due to the paucity of data, there are not many qualitative studies comparing all of the various products that are provided. Studies comparing fibrin sealant (FS) to a standard manual compression (MC) shows its purpose in controlling problematic bleeding intraoperatively [48] and reducing time to achieve hemostasis (TTH) [49], although it does not provide a statistically significant decrease in the amount of blood loss, transfusion rate or postoperative bile leakage [50]. Other studies comparing different types of hemostasis show a faster time of TTH in combined types of TA [51] or that both collagen and fibrin TA could be used with similar effects [52], although still with no difference in terms of blood loss or transfusion rate [53].
Conducted meta-analyses confirm that use of TA is rational to manage intraoperative bleeding [54] and significantly reduces TTH [55] but with no effect on BL, TR or postoperative drainage. TA application helps achieving proper hemostasis intraoperatively, especially in scarce situations, by notably decreasing TTH, and may be a useful adjunct when needed; nevertheless they have no impact on intraoperative BL, TR or perioperative outcomes [56] and are quite expensive in everyday practice [57]. However, they are widely used to manage dire intraoperative situations such as severe bleeding and to provide intraoperative hemostasis.
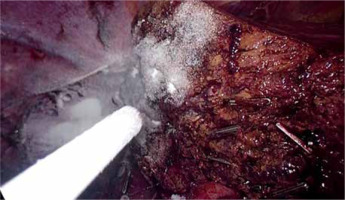
Nonetheless, in LLS, application of some TA may be challenging. Although there are no data addressing this issue, according to the clinical experience, sprays and powders are easier to apply via laparoscopy than sponge-based local TA (Photo 8).
In conclusion, topical agents are a helpful tool in managing difficult intraoperative situations, such as unexpected severe bleeding, and should be at hand when planning major surgical procedures with expected possible intraoperative severe bleeding. However, according to the current data, they have no significant impact on blood loss and transfusion rate.
Anesthesia
In surgical procedures, especially in laparoscopy, perioperative care and preparation of the patient and other non-operative management have significant importance. Even with the huge development of various surgical techniques and equipment, blood loss in hepatic resection remains a massive concern. Therefore a variety of non-operative methods have been developed to decrease intraoperative blood loss and transfusion rate, as well as complication rate, length of stay, morbidity and mortality in hepatic resections [36].
Intraoperatively, maintaining low central venous pressure (CVP) is a main concern. CVP measures volume status and its low level decreases potential BL during the surgical procedure. Low CVP helps significantly reducing intraoperative BL [58, 59] and its value should be under 5 mm Hg, optimally 2.1–3 mm Hg [60]. Stroke volume variation (SVV) is another method of intraoperative monitoring of patients’ fluid responsiveness and can be used without a central venous catheter, measured through an arterial line. SVV may be considered as an alternative to CVP monitoring, with its value needing to be over 10% [61]. Some research indicates lower BL in the SVV group in LLS [62] as well as in liver transplantation [63], but combination of CVP and SVV methods may become a future standard in liver surgery [58].
There are various techniques to reduce hepatic venous congestion and therefore intraoperative BL, the main one being restrictive fluid therapy. Both absolute fluid restriction and relative volume redistribution are comparable techniques, as long as low CVP or high SVV is achieved [59]. Goal-directed fluid therapy, suited individually to the patient, is a recommended approach [64]. To pharmacologically control low CVP during LLS anesthesia, milrinone may be a better choice than nitroglycerin, reducing intraoperative BL, but there are no data related to LLS [65]. Other research confirms decreased BL and TR when using terlipressin infusion to maintain low CVP [66]. The reverse Trendelenburg position, used commonly in LLS, also helps to reduce intraoperative BL (Photos 9 and 10) [67]. Pneumoperitoneum, with its overall positive effects on reducing intraoperative BL, should be between 10 and 14 mm Hg [68], but it could be temporarily increased in dire situations to help control bleeding during LLS [69].
For LLS, general endotracheal anesthesia is preferred, as in other laparoscopic procedures. However, usage of epidural anesthesia may benefit in terms of decreased pain after the operation. It also helps achieving low CVP and therefore reducing BL [70]. Moreover, in terms of analgesia, LLS is associated with less use of narcotics than open surgery [71].
Mechanical ventilation using low tidal volume may reduce bleeding during LLS when compared to conventional tidal volume, but due to the lack of data further studies are needed [72]. Low airway pressure (AWP) during anesthesia helps to control intraoperative bleeding from the hepatic veins and it is safer than increasing pneumoperitoneal pressure [73]. Even a brief pause in mechanical ventilation may be considered to reduce blood loss when facing massive bleeding during LLS [74]. However, more non-experimental studies are needed to confirm these findings.
Acute normovolemic hemodilution (ANH) is a blood conservation technique, which involves preoperative removal of a portion of the patient’s blood, filling the patient’s volume with crystalloids to maintain normovolemia and autogenic transfusion of the patient’s blood after the surgical procedure. Widely used in urology, cardiac surgery and orthopedics, it has a role in major hepatic surgery, reducing TR. However, this technique is useful only in certain cases, when extensive blood loss is presumed; therefore, further attempts are needed to select the patient group which will most likely benefit from ANH [75]. Another procedure like ANH is hypovolemic phlebotomy (HP), which involves removing a portion of the patient’s blood without replacing it. It results in a 10–12% reduction of circulating blood volume. Combined with CVP reduction, this technique lowers bleeding during parenchymal liver transection, resulting in lower TR, but with no impact on intraoperative BL. Nevertheless, due to the data paucity, further RCTs are needed to clarify this method in LLS [76]. In the current situation, these methods are reserved only for selected specific cases. As in other abdominal procedures, in LLS mild perioperative hypothermia reduces intraoperative BL [77, 78].
To summarize, maintaining low CVP or high SVV in LLS significantly helps reducing blood loss. It can be achieved by various methods, including proper patient positioning, fluid restriction, pneumoperitoneum between 10 and 14 mm Hg and reducing AWP to control bleeding from the hepatic veins. ANH and HP may be useful only in selected, specific cases.
Conclusions
Laparoscopic liver surgery has gained popularity in recent years and is becoming a standard practice in more and more surgical wards in scheduled surgery [79, 80]. Despite its higher procedure cost when compared to open liver surgery, because of the lower rate of postoperative complications and shorter hospitalization time in LLS, total costs are similar in both types of procedures [81, 82]. LLS results in reduced blood loss and transfusion rate, as well as in lower morbidity and shorter hospital stay [83, 84].
Transection devices, such as harmonic scalpels and ultrasonic aspirators, help maintain good hemostasis and work best combined together with other tools such as bipolar cautery or harmonic scalpels. Moreover, ultrasonic devices are becoming essential in major liver surgery.
Topical agents are helpful in managing dire intraoperative situations, especially unexpected bleeding, yet, according to the recent data, they have no significant impact on BL and TR.
The Pringle maneuver, widely used in open liver surgery, also has a role in LLS. Types and techniques of PM differ depending on surgeons’ preferences. However, more prospective studies are needed to evaluate its impact on postoperative blood loss and transfusion rate.
In LLS, proper anesthesia, maintaining low CVP or high SVV, as well as proper patient positioning and goal-directed fluid therapy, are of the highest importance, resulting in lower BL.
The difficulty of the procedure, especially in the case of problematic location of lesions, slows down rapid diffusion of LLS. However, proper case difficulty assessment in accordance to surgeons’ experience may decrease the risk of excessive intraoperative bleeding. Difficulty scales, such as IMM or IWATE criteria, help in training hepatobiliary surgeons by focusing on specific skills in selected cases, which may result in better outcomes of LLS in the future.
In conclusion, laparoscopic liver surgery is a fast-developing branch of surgery with plenty of techniques and devices that are helpful in providing good hemostasis with a lot of new possibilities to be used and developed in the future.