Introduction
To this day surgical aortic valve replacement (sAVR) represents a first class recommendation for the management of severe aortic valve pathologies in the majority of symptomatic and all asymptomatic patients with indications (class I, level B) [1]. While correlating with a low risk of early mortality (2.6–3.2%) and morbidity (1.4–1.9%) conventional AVR performed through full sternotomy (FSAVR) has become the most frequently used surgical procedure for heart valve pathologies, as it offers unrestricted access to the heart and the great vessels. Therefore, it has reflected a reference approach for sAVR [2]. However, full sternotomy remains an invasive method, which negatively affects the global postoperative state or preferably the “systemic homeostasis” of each individual patient. It is expressed as a complex reaction of the organism, reflected in systemic organ activities, motoric abilities and mental perceptions. Based on the correct assumptions, the real impact of surgical invasiveness on the patient’s clinical condition appears thus multidimensional.
The collective term “minimally invasive cardiac surgery”, which represents an innovative idea to modify treatment effects by limiting the extent of the surgical cut, was introduced in the mid-1990s. Many attempts have been made to develop novel, less aggressive operative techniques, which essentially focused on the avoidance of full sternotomy. Numerous types and modifications of mini-invasive approaches were described, with J-shaped ministernotomy being most widely adopted [3]. A multitude of published small-sampled randomized control trials [4, 5] and meta-analyses [6–8] provided evidence for safety and non-inferiority of minimally invasive aortic valve replacement (MIAVR). However, crucial estimations of its efficacy, potential advantages and limitations have not been determined yet, leaving freedom to interpret the results sometimes mutually exclusive.
Aim
The aim of this study was to assess the safety and effectiveness of MIAVR by comparing short- and long-term outcomes to reference FSAVR. Complex propensity score (PS) analysis has been incorporated to allow valid comparison and correct inference.
Material and methods
Inclusion and exclusion criteria
This study was based on consecutively collected data of the patients who underwent isolated surgical AVR at our institution between January 2004 January 2018. Patients aged < 18 years and those requiring concomitant treatment of other heart valve, coronary artery disease and ascending aortic aneurysm were not included in the study. Previous cardiac surgery, salvage procedure, active endocarditis, left ventricular end-diastolic diameter (LVEDD) > 80 mm, left ventricular ejection fraction (LVEF) < 18% were also considered as exclusion criteria. Finally, 2386 patients were included in the analysis, in whom two different surgical approaches have been applied.
Surgical techniques used
FSAVR
Transesophageal echocardiography (TEE) was routinely applied before the procedure to confirm indications and to establish the operative strategy. The sternum was divided midline from the sternoclavicular junction to the xiphoid appendage. Total pericardiotomy was performed and extracorporeal circulation (ECC) was set up centrally. A venting line was inserted through the right superior pulmonary vein. Moderate hypothermia (32°C) was used. Repeated, cold, bloody cardioplegia was administered antegradely to the aortic bulb or directly to the coronary ostia in the presence of significant aortic insufficiency. Additional retrograde perfusion via the coronary sinus was utilized in 68% of cases. A new prosthesis was implanted in a continuous manner using 2.0 monofilament sutures. After weaning cardiopulmonary bypass (CPB), ventricular pacing wires and chest drains were placed. TEE examination was used to evaluate procedural results.
MIAVR
All procedural aspects were similar with the conventional method, but some distinguished modifications need mentioning. External defibrillating pads were always applied before the procedure. After the 5–7 cm skin incision, an upper J-shaped hemi-sternotomy from the sternoclavicular junction to the level of the third or fourth intercostal space was performed with RIMA preservation. Partial upper pericardiotomy was done and direct central cannulation was performed for ECC. The venting line was inserted into the pulmonary trunk. Only antegrade administration of cardioplegic solution was utilized. Safe placement of epicardium pacing wires needed the heart to stay unfilled. It was done before aortic de-clamping. Continuous insufflation of carbon dioxide and TEE guidance facilitated the air removal process. Before weaning CPB, a flexible mediastinal Blake’s drain was inserted into the pericardium through the previously tunneled retrosternal space.
Preoperative baseline characteristics
Twenty-two patient-dependent factors were used to construct our PS model. All pre-operative characteristics are presented in Table I. Due to the different development of our mini-invasive aortic program over time, the analysis took into account the δ factor, which determined the period in which the operation was performed.
Table I
Preoperative baseline characteristics before and after 1:1 PS matching. Forced matching variables are marked in gray
[i] MIAVR – minimally invasive aortic valve replacement, FSAVR – full sternotomy aortic valve replacement, BMI – body mass index, MI – myocardial infarction, PCI – percutaneous coronary intervention, PAOD – peripheral arterial occlusive disease, LVEF – left ventricular ejection fraction, LVEDD – left ventricular end-diastolic diameter, NYHA – New York Heart Association Functional Classification, EuroSCORE – European System for Cardiac Operative Risk Evaluation, “δ” (delta time variable) – patient-related covariate coding the date of surgery. Urgency [emergency case. admission directly from external cardiology department, symptomatic patient with 1 month referral]; Pulmonary disease [COPD-chronic obstructive pulmonary disease, bronchial asthma, emphysema {radiological lesions combined with significant spirometry changes: FEV1/VC < 70%}]; chronic kidney disease [G3 category or higher: GFR < 50 ml/min/1.72 m2]; impaired mobility [various neurological or skeletomuscular conditions contributing to mobility limitation that may affect the process of proper wound healing and rehabilitation]; “δ” variable [determining the time elapsed from the date of surgery to the moment of analysis given the integer coded a six-month time interval].
Primary and secondary outcomes
Main treatment effects: 1) in-hospital mortality; 2) all-cause reoperation rate; 3) early neurological events (new neurological deficits: stroke, transient ischemic attack – TIA, delirium, global anoxemic brain injury); 4) early cardiac events (perioperative myocardial infarction – PMI, myocardial ischemia – MI, malignant ventricular arrhythmias); 5) pulmonary complications (confirmed by X-ray, USG or CT scans); 6) wound healing complications (superficial and profound type).
Secondary events: (all events of interest are listed in Table II) ~ intraoperative measures, secondary postoperative in-hospital outcomes
Table II
Primary main events, secondary intraoperative and postoperative outcomes from the PS sample (n = 1114) and from the total sAVR data after multivariable adjustment for the PS (n = 2386)
[i] MIAVR – minimally invasive aortic valve replacement, FSAVR – full sternotomy aortic valve replacement, TIA – transient ischemic attack, PMI – perioperative myocardial infarction, MI – perioperative myocardial ischemia, VF – ventricular fibrillation, IABP – intra aortic balloon pump, AF – atrial fibrillation, SWI – superficial wound infection, VAC – vacuum assisted wound closure, CPB – cardio-pulmonary by-pass, C-C – cross-clamp, RBC – red blood cells units transfused, ICU – intensive care unit, OR – odds ratio, DifM – difference in means. p (*) {dyadic structure assumed} – binominal McNemar exact test [binary]; Paired T-test [parametric]; Wilcoxon signed rank test [nonparametric].
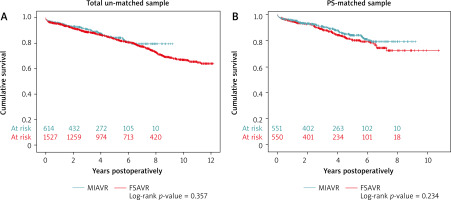
Long-term results: 1-, 5- and 8-year survivals. Kaplan-Meier curves have been plotted for comparison.
Study design
In accordance with the recently published ESCTS guidelines for PS-based study management [9], the current analysis was performed in the following steps:
PS estimation and matching – A logistic regression was adopted to estimate the specific PScore for each individual sAVR patient. All predictors shown in Table I were included. As recommended, the subjects have been grouped on the estimated logit-transformed PScore by means of 1 : 1 treated-control matching. A “greedy” algorithm on the Mahalanobis distance was used. No matches outside the caliper width of 0.2 σ were allowed. Importantly, forced matching on the specified variables was done to minimize the predictor-outcome effect.
Baseline covariate balance assessment – Three global multivariate approaches to measure imbalance were used. They required additional RITOOLS software and the CEM application programmable in R language:
The z-difference (difference in means/proportions divided by SE). Simplicity, generality, graphical accessibility and summarizability made it recommended for PS-paired analyses [10].
The overall imbalance d2 test (Hansen & Bowers). Based on the latently randomized experiment, the d2 test verified the hypothesis of null imbalance before and after matching [11].
The L1 imbalance measure (Iacus, King & Porro). It was derived to assess a full joint distribution of the L1 difference between the two multidimensional histograms of all predictors, being coarsened already into custom bins [12].
Analysis of outcomes – Assuming no independence of the dyadic PS data structure, only the adjusted regressive models that controlled for all covariates used in matching were adopted. Conditional logistic regression and mixed-effects linear model giving odds ratio (OR) and difference in means (DifM) with 95% confidence intervals (95%CI) were addressed for binary and continuous outcomes, respectively. The McNemar binominal exact test for binary variables and paired t-test or Wilcoxon signed rank test for scale measures were carried out in parallel. Time-to-event data in long-term observation were analyzed by Kaplan-Meier estimation. The log rank test was proposed for survival comparison. Since the PS matching could exclude some portion of unmatched cases from further evaluation, a second method of covariate adjustment for the PScore was applied to confirm the reliability of results. For this task, multivariate logistic regression and general linear model were used.
Defining the quantity of interest – The estimand represented the average treatment effect for the people who were matched. Fisher’s exact test and the Mann-Whitney U test were used to characterize the unmatched people.
Results
Forced 1 : 1 PS matching
Out of a total of 2386 patients, 620 underwent minimally invasive AVR (MIAVR) and 1766 full sternotomy AVR (FSAVR). The final fit PS model created 557 well-allocated pairs of treatment (MIAVR) and control (FSAVR) units who presented identical gender and the type of aortic disease (Table I).
Global balance testing
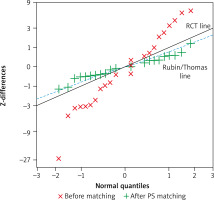
A smaller individual z-difference value showed significant reduction of imbalance for each preoperative covariate in the matched subset. After matching the distribution of z-differences for all predictors overlapped the reference “Rubin/Thomas” line, approximating to the standard for a randomized study. Graphical representation of the allocation of each individual covariate is shown in Figure 1. Considering that SEM for the 27 z-differences was equal to 1/√27, the estimated mean of z-differences was –0.81; 95% CI [–1.19; –0.44] before matching and –0.03; 95% CI (–0.41; 0.35) in our thoroughly balanced PS sample, similar to the characteristics of a normal distribution.
Figure 1
Q-Q plot for the z-differences of preoperative predictors before and after 1 : 1 PS matching. The solid grey line corresponds to the expected distribution of z-differences from a randomized trial, the broken blue line corresponds to the “Rubin/Thomas line” for z-differences from a perfectly matched PS analysis
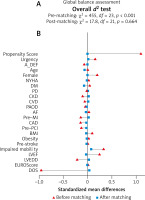
The omnibus d2-test assessed the overall χ2 imbalance on all of the linear combinations of all predictors. While rejecting the null hypothesis in the unadjusted data, the d2 test confirmed no imbalance for our PS-matched observations (Figure 2).
Figure 2
Multivariate global balance testing with randomization inference (A). Balance plot for the standardized differences (biases) from the set of pre-operative covariates before adjustment (red) and after 1 : 1 PS-matching (blue) (B)
The overall balance assumption was provided in the form of the L1 relative measure. The multivariate imbalance test showed the improvement of global imbalance in the PS-matched population (L1 = 0.770) in relation to the total sAVR prematchings (L1 = 0.862).
Treatment effect size
Effectiveness of the intended mini-invasive approach
Out of 620 MIAVR cases, 14 (2.3%) patients required conversion from ministernotomy to full sternotomy intraoperatively. Three of them occurred before aortic clamping, because adequate exposure could not be achieved, and the next two happened after aortic clamping due to unusual patient’s anatomy. The majority (64%) of converted cases appeared after aortic de-clamping as a consequence of sudden hemorrhage, which followed RV perforation, aortic root damage, left main injury, significant perivalvular leak or left ventricle distention impossible to defibrillate.
Main treatment effects
In-hospital mortality was low and similar in both groups with the rates of 1.26% for MIAVR and 1.62% for FSAVR (Table II). No differences in terms of total reoperation rate or cardiac and pulmonary complications were found. Non-significantly lower neurological risk was observed for MIAVR patients (OR = 0.72; p = 0.09). Nevertheless, this non-significant difference ultimately reached statistical significance when analyzing the total sAVR population (OR = 0.67; p = 0.02). Furthermore, MIAVR patients had a 24% lower risk of complicated wound healing in relation to FSAVR individuals (OR = 0.31; p < 0.001).
Secondary events of interest
Even though no difference was found with respect to re-explorations for bleeding or tamponade, most cardiac, pulmonary and renal complications showed comparable occurrences. Yet, there were non-significantly reduced risks of early stroke (OR = 0.50; p = 0.1) and LOS (OR = 0.66; p = 0.13) in the MIAVR group. Moreover, shorter intubation time (DifM: –6.9 h, p < 0.001), shorter ICU stay (DifM: –8.32h, p = 0.02), reduced 24-h postoperative blood loss (DifM: –96.56 ml, p < 0.001) and fewer complementary blood transfusions (DifM: –0.57 U, p < 0.001) were found as attractive outcomes in favor of MIAVR (Table II).
PS-adjusted analysis of the total sAVR population
Multivariate regressive models confirmed the superior results of MIAVR over FSAVR. MIAVR receivers were 40% less likely to develop neurological complications (OR = 0.67; p = 0.02) and postoperative psychosis (OR = 0.66; p = 0.030than FSAVR patients. A clearly decreased risk of LOS (OR = 0.45; p < 0.001) was observed in the MIAVR group, confirming the trends observed in the PS-matched sample (Table II).
Long-term results
Kaplan-Meier actuarial analysis revealed no significant difference between the two surgical approaches in terms of long-term survival. Expected 1-, 5- and 8-year survival rates were comparable, reaching 95.6% vs 94.8%; 84.1% vs 80.4% and 79.3% vs 72.4%, for MIAVR vs FSAVR, respectively. Referring to the total sAVR before matching, no pronounced changes were observed either (Figure 3).
Local” SATT – the sample average treatment effect on the treated
The estimand
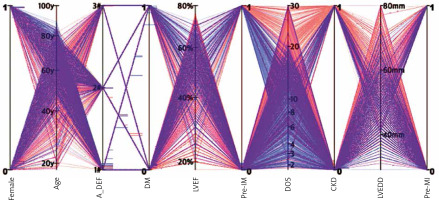
Visual representation of the patients who were matched (“local” SATT) was obtained via a parallel coordinates plot (Figure 4). The PS-matched population consisted of a greater percentage of females, predominance of aortic stenosis, higher incidence of diabetes and impaired mobility, and more preserved LVEF. A lower incidence of chronic kidney disease, coronary artery disease, previous myocardial infarct and smaller LV characterized the estimand.
The unmatched
As many as 68% of the FSAVR patients were not paired. It is worth noting that they were mostly operated on before 2010. Since forced matching was used, 63 MIAVR individuals remained unmatchable because no appropriate control matches could be found. Nonetheless, they showed at least as favorable results as the MIAVR matches, revealing concurrently a much shorter overall hospitalization time. Analysis of outcomes in this subgroup confirmed the reliability of the results, thus allowing their generalization to the entire sAVR population (Table III).
Table III
Primary main events, secondary intraoperative and postoperative outcomes of patients excluded from the PS analysis – “the unmatches” (n = 1272)
[i] Abbreviations: see Table II; FET – Fisher exact test (binary), UMW – U Mann-Whitney test (continuous).
Discussion
The main intention of this study was to estimate the unbiased treatment effect size. Thus, our PS clinical research aimed at reducing imbalances between the two compared groups. The application of such a “balancing score” in combination with conditional regressive approaches for outcomes analysis corrected the effects of channeling bias and potential confounders, allowing results close to randomization to be obtained.
The current study demonstrated that MIAVR was a safe, effective and reproducible procedure providing good short- and long-term results comparable to conventional FSAVR. However, MIAVR patients showed a significantly shorter time to extubation and discharge from the ICU, and an improved blood conservation strategy following clearly less 24-hours postoperative drainage. Moreover, this analysis has proven that ministernotomy decreased the risk of impaired wound healing. In addition to a lower chance of having LOS postoperatively, the cerebrovascular risk was reduced for MIAVR when compared to FSAVR.
“Safety first!”
Despite our sAVR population showing a relatively high preoperative risk profile, low in-hospital mortality was observed in both groups. Overall, the estimated results were consistent with most reports [13–18]. Admittedly, the PS study designed by Merk et al. [19] showed a significantly lower 30-day mortality (0.4% vs. 2.3%; p = 0.01) and better 5- and 8-year survival (p = 0.03) in favor of MIAVR. Those findings remained unexplainable, although a rather large number of premature deaths was noted in their control group, potentially affecting the outcomes. It was agreed that providing proof of the superiority of one technique over another was very difficult to achieve, due to sample size requirements. Yet, since our follow-up Kaplan-Meier estimation revealed no significant differences in 1-, 5- and 8-year survival rates between MIAVR and FSAVR (Figure 3), the operative approach should definitely not be considered a negative predictor of late mortality. Certainly, we were unanimous with other colleagues in this matter [18].
In our series, the success of the intended ministernotomy approach reached almost 98% with 14 cases requiring intraoperative conversion to full sternotomy. Published data estimated the conversion rate from 2.6% to 4.0% [6, 7, 20]. Contrary to Cleveland Clinic experience [20], the majority of our cases were converted after aortic de-clamping, correlating thereby with a much higher mortality in this subgroup (14.3%). Also worth noting was the number of secondary conversions during re-explorations for bleeding or tamponade, which has been constantly ignored by most researchers. Over 70% of our 37 re-explored MIAVR patients were not converted and the ministernotomy approach could be successfully maintained until discharge. Although the frequency of re-explorations was the same, its negative impact was particularly more expressed in the mini-invasive group. It resulted directly from mediastinal tightness as a consequence of incomplete pericardiotomy. Such a situation was more likely to occur in obese individuals suffering from pulmonary diseases.
The concept of myocardial protection was a discriminant factor for surgical techniques used. Only antegrade administration of a cardioplegic solution was used in the MIAVR group, while 63% of FSAVR patients had additional retrograde myocardial perfusion. Despite that, the rate of cardiac complications and the need for inotropic support were comparable. Surprisingly, a clearly decreased risk of LOS was detected for MIAVR. Similarly, Shehada et al. [8] in a meta-analysis found reduced incidences of LOS and AF for their MIAVR. These observations made it possible to state that not a route, but the style of cardioplegia delivery was likely to play a much more important role in myocardial preservation. The type of cardioplegia administered, the appropriate volume perfusing the coronary vessels and the time between successive doses together have an essential impact on the extent of cardiac ischemia during surgery.
Taking into consideration restricted visualization, accessibility and the incomplete de-airing process, the ministernotomy approach has become more challenging, especially in the presence of the multiple sclerotic aorta and massive calcification of the native aortic valve. It might seem that MIAVR would correlate with a higher neurological risk. Meanwhile a non-significant tendency of decreased stroke risk by 33% was observed. Likewise, analogous benefits of the mini-invasive strategy have been reported by other authors [6, 7, 13–17, 20, 21]. In contrast to some investigators [8, 16, 18, 22] we did not confirm a protective effect of MIAVR in decreasing the occurrence of AF. It was remarkable, however, how high the frequency of AF detected by 24-h ECG telemetry in both groups was (37.5% vs. 38.1%, respectively), despite complex postoperative management aimed at avoiding such a complication. These realistic numbers have been sending a serious warning signal to the clinicians, raising reasonable questions about the necessity of anticoagulant therapy for biological valve receivers in the early postoperative period.
“The patient above all!”
Advantageously, MIAVR patients had a 6.9 hours shorter time to extubation and an 8.3 hours shorter ICU stay than FSAVR participants. Various reports have confirmed our findings on this issue [7, 18]. The meta-analysis by Brown et al. [6] provided strong evidence for a correlation of the minimally invasive approach with faster time to be extubated (WMD: –2.1 h; p < 0.01) and discharged from the ICU (WMD: –0.72 d; p < 0.01). Similarly, less surgical invasiveness should have a positive impact on postoperative blood loss and the need for complementary transfusions [6, 20, 21]. Indeed, significantly decreased 24-hours chest drainage and a smaller amount of blood units transfused were detected in our MIAVR series. Also, MIAVR distinctively decreased the risk of superficial wound infections, and so the odds of improper wound healing were reduced by 82% in relation to FSAVR. Importantly, most wound restoration problems occurred more frequently in the diabetic and obese individuals. Unlike some researchers [17], it was actually in the case of obesity that the minimally invasive option was requested first at our department. These noticeable advantages, however, reflected the role of better sternal stability in respiratory function preservation and mobility improvement. All the features jointly contributed to faster recovery, which appeared to be the best advantage of ministernotomy.
Searching for reasons of our fatal courses revealed that the MIAVR failures were correlated with the elderly patients who died of acute tamponade diagnosed too late. In contrast, most of the FSAVR failures referred to females, obesity, kidney disease, impaired mobility, prolonged CPB and LOS. Hence, obese diabetic females with chronic pulmonary diseases and impaired mobility would probably be better candidates for the minimally invasive strategy instead of cardiomyopathic octogenarians with the multiple sclerotized aorta, a small massively calcified aortic valve and several comorbidities, in whom TAVI interventions should now be considered.
This is a retrospective observational study based on a single-center experience, which could restrict its general inference. The analysis did not include the assessment of postoperative pain, the patient’s quality of life or cost-effectiveness rating. Although a lot of information on the course of early rehabilitation has been collected for MIAVR, the lack of sufficient data relative to FSAVR restricted such a comparison. Furthermore, the study could not correct for all unknown and unobserved measures, which only a randomization would have done in fact. Therefore, none of the methods will be able to reproduce the RCT results.
Conclusions
Minimally invasive aortic valve replacement through J-shaped ministernotomy has proven to provide short- and long-term survival comparable with reference conventional AVR.
Nevertheless, MIAVR should be recommended to obese, diabetic patients with concomitant lung diseases and mobility disorders to facilitate their rehabilitation process, to support proper sternal wound healing and to provide faster recovery. The protective feature of ministernotomy against neurological complications allows MIAVR to be considered in patients with high cerebrovascular risk, yet the indication requires further evaluation. Complementary, well-designed studies considering late mortality and morbidity are definitely needed to establish the guidelines for minimally invasive AVR in the surgical management of aortic valve pathologies.