Rheumatic heart disease (RHD) nowadays has become exceptionally rare in industrialized countries [1]. At the same time, in developing nations, RHD remains a leading cause of morbidity and mortality of cardiac origin [1]. Rheumatic tricuspid valve disease (RTVD) is infrequent, occurring in about 7–9% of RHD cases [2]. Patients with hemodynamically significant RTVD requiring surgical correction are extremely rare. They almost always have associated rheumatic mitral valve (MV) pathology [1]. Existing experience in the management of RTVD is largely based on old studies dating back to the last century [1]. Issues regarding indications, optimal timing, and method of surgical correction of RTVD are still under debate [2]. Global migration of the population explains the influx of patients with RHD and the possibility of their presentation for medical treatment to any hospital in the world. We present a case of successful operative management of mixed RTVD associated with mitral stenosis (MS).
A 59-year-old woman was admitted to the hospital with complaints of reduced exertional capacity and dyspnea. The patient had heart failure of Class III in the New York Heart Association (NYHA) classification. She was first diagnosed with RHD 26 years ago. At the age of 35 years, she underwent a closed mitral commissurotomy for severe MS.
Upon objective examination leg edema, ascites, hepatic congestion, and Kussmaul’s sign were noted. In auscultation, there was a high-pitched holosystolic murmur and low-frequency mid-diastolic murmur in the left fourth intercostal space, indicating combined tricuspid insufficiency and stenosis. A loud first heart sound, an opening snap, and a diastolic rumble were heard loudest at the 5th intercostal space on the midclavicular line, indicating a MS. ECG demonstrated a sinus rhythm and was significant for P-pulmonale and tall and peaked T waves in II, III, and aVF. Laboratory analysis showed elevated liver enzymes (AST 152 IU/l; ALT 175 IU/l).
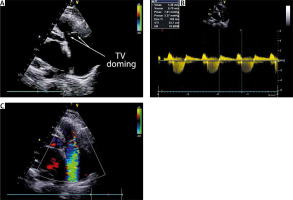
Transthoracic echocardiography (TTE) was significant for left atrium (LA) and right atrium (RA) enlargement. LA diameter 5.8 cm, LA volume 65 ml. RA size 63x41 mm. Left ventricle (LV) ejection fraction (EF) was 53%. MV leaflets and subvalvular structures were thickened, fibrotic, and fused in commissures; the posterior leaflet (PL) was limited in mobility. MV area 1.1 cm2; maximal pressure gradient (PG) 11 mm Hg. Moderate regurgitation. Tricuspid valve (TV) leaflets were diffusely thickened and fused in commissures with doming of the valve. TV area 1.4 cm2; maximal PG 7.6 mm Hg; inflow time-velocity integral 53.7 cm; T½ 158 ms; severe regurgitation; tricuspid annular plane systolic excursion (TAPSE) 21 mm (Figure 1). Pulmonary artery (PA) pressure 40 mm Hg. Coronary angiography was normal.
Figure 1
Echocardiographic presentation of the rheumatic tricuspid valve (TV): A – 2-dimensional modified apical four-chamber image demonstrating doming of the TV; B – modified apical fourchamber image with continuous wave Doppler through the TV demonstrating TV stenosis; C – apical four-chamber image demonstrating TV insufficiency
The patient was referred for surgical treatment: mitral and tricuspid valve replacement.
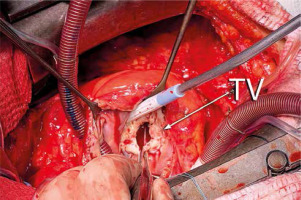
The operative approach was through a midline sternotomy. The heart was freed from adhesions. A standard aortic and bicaval cannulation was used for initiation of the cardiopulmonary bypass (CPB). Mildly hypothermic (32°C) intermittent cross-clamp fibrillation (ICCF) was used for myocardial protection. The MV was approached through right atriotomy and the interatrial septum. Upon inspection, a severely malformed rheumatic MV was revealed. It was replaced with a Masters Series No. 29 mechanical prosthesis (Abbott, USA). The MV posterior leaflet was preserved. TV leaflets were diffusely thickened and fused in commissures (Figure 2). The TV was replaced with an Epic No.31 biological prosthesis (Abbott, USA). The operation was completed in a standard fashion, and the patient was transferred to the intensive care unit (ICU) on minimal inotropic support. Her postoperative course was uneventful. The ICU stay was 2 days, and the postoperative hospital stay was 8 days.
Figure 2
Intraoperative presentation of the tricuspid valve (TV). Leaflets are diffusely thickened and fused in commissures
At the 6-month follow-up at the 0.5 year follow-up, the patient was in a good clinical condition, NYHA Class I. Her TTE demonstrated a good mitral and tricuspid prostheses function, improved LV contractility (EF 63%), decreased LA and RA volumes, and PA pressure (25 mm Hg).
Although RHD is almost eradicated among the population of industrialized countries, it is still one of the leading causes of morbidity and mortality in the developing world. RHD is responsible for 233 000 deaths annually [1].
Primary right heart valve disease is infrequent. According to the Euro Heart Survey, it is found in 1.2% of patients with cardiac valve pathology [3]. Up to a third of these cases are caused by RHD. Half of them occur in the form of an isolated tricuspid regurgitation, and another half as combined TV stenosis and regurgitation [2].
Differential diagnosis should be performed with carcinoid syndrome, systemic lupus erythematosus, and antiphospholipid antibody syndrome [4]. Iatrogenic effects of the fusion of the permanent pacing lead with TV leaflets and subvalvular apparatus may lead to TV malformation [5]. Congenital defects, such as Ebstein’s anomaly, Fabry’s, and Whipple’s diseases, are also among the etiologic factors [6].
Almost always, RTVD is associated with other heart valve diseases. In the majority of cases, it is MS, as was the case with our patient [2]. Standards for diagnosis, indications for surgery, and methods of operative correction are well established for rheumatic mitral and aortic valve pathology. In regard to RTVD, these questions are still under debate due to the rarity of the pathology and the lack of large-scale research [1, 2].
Our patient’s clinical presentation and physical examination data were typical for severe MS. TTE has confirmed this diagnosis. Rheumatic moderate-to-severe tricuspid stenosis combined with severe tricuspid regurgitation was an unusual finding. This fact stresses the importance of meticulous TV examination during TTE in every patient with RHD. Doming of the TV leaflets, as was detected in our patient, is reported to be the most valuable criterion for diagnosing TS with specificity and sensitivity of 100% [2].
Our patient was qualified for surgery based on current recommendations for the management of severe MS and associated severe tricuspid regurgitation combined with moderate TS [7]. ICCF was used for myocardial protection as it is our clinical routine in isolated mitral and tricuspid valve procedures. Hemodynamic conditions between the left and right heart chambers are different in terms of blood flow velocity, intracavitary pressures, and transvalvular pressure gradients. Therefore the choice between biological and mechanical prostheses for the mitral and tricuspid valve replacement required a more differentiated approach than if it were for the left-sided mitral and aortic valves. The patient’s MV valve was replaced with a mechanical prosthesis because of her relatively young age. This decision carries potential long-term survival benefit in this case. Pathoanatomical changes in the TV, a combination of severe regurgitation and stenosis, favored TV replacement rather than repair. The incidence of thrombosis of the mechanical prosthesis in the tricuspid position is as high as 20%, while in the mitral position it is reported to vary from 0.1% to 6% [8, 9]. Probably, this can be explained by the lower blood flow velocity across the prosthesis in the tricuspid position. In order to avoid this life-threatening complication, the biological prosthesis was selected as a tricuspid valve substitute in our patient [10]. In case of potential biological valve structural degeneration in the future, a repeat surgical tricuspid valve replacement and transcatheter valve-in-valve procedure remain a possible solution in this case [11].
Surgical correction of RTVD is a high-risk procedure. Reported operative mortality rates vary between 8% and 28% [1]. It may be due to a number of factors. Patients in this subgroup have often had previous surgery. Their clinical condition is poor due to cardiac insufficiency and multiple comorbidities. They almost always require concomitant surgery on the mitral valve. Low cardiac output may complicate their postoperative period due to right ventricular dysfunction, especially in cases with delayed presentation [2]. Our case was a relatively young female with hemodynamically significant rheumatic mitral and tricuspid valve disease after a previous closed mitral commissurotomy. She was in NYHA heart failure Class III and had liver dysfunction. She successfully underwent mitral and tricuspid valve replacement. Her immediate and early postoperative period was uneventful. We attribute it to the timely surgery that was performed before the left and right ventricular function significantly deteriorated. Mitral and tricuspid valve replacement provided for immediate improvement of the intracardiac hemodynamics, which also contributed to the successful result of the treatment.
In conclusion, although rare, cases of rheumatic disease of the tricuspid valve may occur in clinical practice. TTE is an optimal tool for the detection of this pathology. Rheumatic mitral valve disease is a permanent accompanying pathology. Surgery should be performed before the left and right ventricle function is severely depressed. The operation must provide for the uncompromising hemodynamic resolution of the pathology.






