Introduction
Protein phosphatases (PP) and kinase are critical for the regulation of many biological functions [1]. Protein phosphatase 1 (PP1) is a member of the serine/threonine phosphatases family and has been reported as highly conserved among eukaryotes [2]. In the cellular system, the nuclear region has been reported as a highly dynamic compartment, and phosphorylation of protein plays an essential regulatory role in signalling pathways, including apoptosis [3]. Earlier reports revealed enriched PP1 activity in the cell’s nucleus and crucial roles associated with the segregation of chromosomes. The localization patterns of PP1-isoforms are dynamic, and their distribution keeps changing throughout the cell cycle and in response to other cellular distresses [4]. The significance and role of the different PP1 isoforms and their association with mitotic events remain unclear, but they have distinct subcellular localization [5, 6].
Recent reports also indicate that PP1 [7] and PP2A [8] are required for mitotic exit. The PP1 isoforms play a crucial role in mitosis in mammals based on their localization pattern in the nucleus in G1 and S phase cells [2]. PP1 consists of 4 isoforms: PP1α, PP1β/δ, PP1γ1, and PP1γ2. While PP1γ1 expresses ubiquitously, PP1γ2 is sperm-specific and essential for spermatogenesis [9]. PP1α is localized to the centrosome, PP1γ1 is associated with the mitotic spindle’s microtubules, and PP1δ is strongly associated with chromosomes during mitosis [5]. PP1γ1 has been reported to play an essential role in cell cycle regulation, and its localization pattern has been correlated with the various stages of the cell cycle [10]. PP1γ1 and PP1γ2 are alternative splice variants, coded by [11] the PPP1cc gene. The PPP1cc gene is known to be associated with many biological processes including regulation of circadian rhythm [12] as well as cell cycle and cell division, glycogen metabolic process, and neuronal differentiation through protein dephosphorylation. Both the isoforms are identical in all respects, except that PP1γ2 has a unique 23-amino-acid carboxyl-terminal extension coded by an extra exon at the 3’ terminal of this gene. So far, the expression of the PP1γ2 isoform has been reported to be testis-specific and plays a critical role in sperm maturation [9]. Simultaneously, several reports have reported that massive expression of germ cell-specific antigens (proteins) is a hallmark of cancerous cells [13]. However, the role of the PP1γ2 isoform concerning cell cycle regulation or other key biological processes is not clear and remains unexplored. Hence, this study was designed to observe the profile of PP1γ2 in the cervical cancer cell line of HeLa cells . In vitro experiments were conducted to better understand the role of PP1γ2 isoform in the dynamics of the cell cycle transition associated with tumourigenesis.
Material and methods
Maintenance of cell line
HeLa cells were obtained from ATCC (Manassas, VA) and maintained in the lab as per the standard protocol. Briefly, the cells were cultured in Eagle’s Minimal Essential Medium (EMEM, M0894, Sigma Aldrich) supplemented with 10% Foetal Bovine Serum (10082147, Gibco) and 1X antibiotic solution (15140122, Gibco) and kept at 37°C in 5% CO2 in an incubator for growth and proliferation. The media was changed every 2–3 days until the required 80–90% confluence was obtained for the cells’ sub-culturing.
RNA isolation and RT-PCR
Total RNA isolation from cells was done using tri reagent (T9424, Sigma Aldrich) as per the manufacturer’s instruction [14]. cDNA synthesis was done with 2 µg of RNA by using a GeneSure First cDNA synthesis kit (PGK162B, Puregene). PP1γ2-specific (NCBI Reference Sequence NM_001244974.2) forward 5’GTGGTTGAAGATGGATATGA3’ and reverse 5’CTGATGCAACCCTTG3’ primers were used with Master Mix (K0171, Fermentas) as per the manufacturer’s instructions. A PCR protocol consisting of an initial denaturation at 94˚C for 2 min, 30 cycles of denaturation for 30 secs at 94˚C, annealing at 43˚C for 30 secs, extension for 1 min at 72˚C, and a final extension at 72˚C for 5 min was used for PP1γ2. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (NCBI Reference Sequence NM_001256799.3) served as an internal control using 5’CACCATCTTCCAGGAGCGAG3’ and 5’TCACGCCACAGTTTCCCGGA3’ primers with an initial denaturation for 2 min at 94˚C, followed by 30 cycles of denaturation at 94˚C for 30 sec and annealing for 30 sec at 54˚C, extension at 72˚C for 1 min, and a final extension for 5 min at 72˚C. Commercially obtained Human Testis RNA (540049, Stratagene) was used as a positive control for PP1γ2 amplification. The products were run on a 2% agarose gel for confirmation of product size.
Protein extraction and quantification
Protein extraction from whole cells was done using RIPA buffer (R0278, Sigma Aldrich) as per the manufacturer’s instructions. Briefly, 100 µL of RIPA buffer containing 1X protease (p8340, Sigma Aldrich) and phosphatase inhibitor (p0044, Sigma Aldrich) was added per 106 cells. After lysis, cells were centrifuged at 14000*g for 10 minutes at 4°C to aspirate the protein-containing supernatant. The NE-PER kit was used (78833, Thermo Scientific) for protein extraction from cytoplasmic and nuclear fractions as per the manufacturer’s instructions. Protein quantification was done by the Bradford method [15] using Bradford Reagent (B6916, Sigma Aldrich).
Specificity of the anti-PP1γ2 antibody
PP1γ2 is a 337 amino acid protein with a molecular mass of around 39 kDa. PP1γ1 and PP1γ2 isoforms are coded as 2 different splice variants of the same gene – PPP1CC. These isoforms are identical except a specific stretch of 23 amino acids at the C-terminal of the PP1γ2 isoform. Therefore, the full-length PP1γ2 protein cannot be explored to get PP1γ2-specific antibodies, and hence a synthetic peptide of only specific stretch of 23 amino acids (VASGLNPSIQKASNYRNNTVLYE) of PP1γ2 was used as an immunogen to obtain affinity-purified anti-PP1γ2-specific antibodies in Rabbit.
Western blotting
Proteins were resolved through SDS-PAGE. Briefly, 25–50 µg of protein lysate was mixed with 2X Laemilli buffers containing β-mercaptoethanol (M6250, Sigma Aldrich), and electrophoresis was performed at a constant voltage (50 V). Proteins were electroblotted on to a nitrocellulose membrane (66485, Pall Corporation) using a semi-dry transfer system (Pierce Power Station, Thermo Scientific), and the membrane was blocked in 0.1% Tween-20 added in phosphate buffer saline (PBST) containing 2% Bovine Serum Albumin (BSA) for 1 hr at room temperature (25°C). The membrane was incubated with the anti-PP1γ2 antibody (1:3000) overnight (12 hrs) at 4°C. After washing, the membrane was incubated with the HRP-conjugated secondary antibody (1:10,000) for 2 hrs at room temperature (25°C). Again, after washing, the blot was developed with Immobilon Western Chemiluminescent HRP Substrate (WBKLSO500, Millipore), and a chemiluminescent signal was measured using ImageQuant™ LAS 4000 gel doc system (GE). Commercially obtained normal human testis lysate (1313, ProSci) was loaded as the positive control.
Flow cytometry analysis of HeLa cells
Confluent cells were harvested and fixed by 4% paraformaldehyde. After washing with PBS, the cells were permeabilized using 0.1% Triton X 100 and 0.05% NP-40 in 1X PBS and washed with PBS. The cells were centrifuged at 300*g for 3 min and incubated with anti-PP1γ2 antibody for 1 hr on ice, followed by washing with PBS. Cells were then incubated with specific fluorescent-tagged secondary antibody for 1 hr on ice in the dark. After washing, the cells were analysed on a BD-FACS-Aria along with all the required controls.
Taxol treatment in HeLa cells
Around 0.1 million cells/well were seeded in 2-chamber polystyrene tissue culture glass slides (BD Biosciences, USA) and kept at 37°C in a 5% CO2 incubator overnight. After the incubation, the media was replaced with a fresh EMEM medium containing 5, 10, 25, 50, and 75 nM Taxol (Paclitaxel, NAPROD lifescience) in replicates and cultured for an additional 2 hrs. These cells were then fixed with 4% paraformaldehyde for 15 minutes at 37°C; after washing with PBS, the cells were processed for the immunofluorescence studies.
Immunofluorescence and confocal microscopy
HeLa cells were seeded on coverslips, fixed and permeabilized as described earlier, and then blocked with 1% BSA for 2 hrs at room temperature (25°C) followed by incubation with PP1γ2 specific primary antibody at a dilution of 1:250 and α-Tubulin (32-2500, Invitrogen)-specific primary antibody at a dilution of 1:500 overnight (16 hrs) at 4°C. After incubation, the cells were washed thrice with PBST and once with PBS, and then probed with polyclonal Cy3 (λex/λem = 550/570 nm)-labelled AffiniPure Donkey Anti-Rabbit IgG secondary antibodies (cat# 711-165-152, Jackson ImmunoResearch Laboratories, Inc.) at a dilution of 1:500 for PP1γ2 expression/localization experiments. FITC (λex/λem = 490/525 nm)-labelled anti-mouse for α-Tubulin and 0.25% DAPI (λex/λem = 490/525 nm) for the nuclear staining were used, and cells were incubated at 37°C in the dark for 2 hours at room temperature. The cells were rewashed as explained above, followed by mounting in Prolong Gold Antifade Reagent (P36934, Invitrogen) and visualized under a confocal laser scanning microscope (Carl Zeiss LSM 510 Meta) using a sequential mode of imaging using a Plan-Apochromat 63x/1.4 Oil DIC M27 objective.
For the red channel (PP1γ2), Cy3 (λex/λem = 555/569 nm) was excited using a 561 nm laser line (DPSS 561-610 laser), and the emission was captured using a BP: 575-615 nm filter. For the green channel (α-Tubulin), FITC (λex/λem = 490/525 nm) was excited using a 488 nm laser line (Multi-line Argon laser) and the emission was captured using BP: 505–550 nm filters. For the blue channel (nucleus), DAPI (λex/λem = 358/461 nm) was excited using 405 nm laser line (Blue Diode Laser) and the emission was captured using BP: 420–480 nm filters.
Exposure of cervical cancer HeLa cells to a hypoxic environment
For hypoxia experiments, cervical cancer HeLa cells were seeded on a 25 cm<sup>2</sup> tissue culture flask until they reached 75% confluence and then exposed to either normoxic or hypoxic conditions for 24 hrs and 48 hrs. In normoxic conditions the cells were maintained at 37°C in a humidified incubator with 5% CO2/95% air. For hypoxia experiments, the culture medium was changed immediately before the cells were exposed to the hypoxic environment within the hypoxia chamber (STEMCELL Technologies, Cambridge, MA, USA), which was maintained at low oxygen tension (1% O2, 5% CO2, and 94% N2). The treatment was initiated by introducing the culture in the hypoxia chamber and replacing the existing culture medium with deoxygenated medium. Deoxygenated medium was prepared before each experiment by equilibrating the medium with a hypoxic gas mixture containing 1% O2, 5% CO2, and 94% N2 at 37°C. At the end of 24 hrs and 48 hrs; no change in media colour was noted, indicating physiologic pH values between 7 and 8 were maintained throughout the experiment. After incubation of up to 48 hrs, cells were harvested, and the pellet was stored at –20°C for further downstream experiments.
siRNA-mediated knockdown of PP1γ2
Two siRNAs specific for PP1γ2 (Si1 sense GCCUGAACCCGUCCAUUCA, antisense UGAAUGGACGGGUUCAGGC Si2: sense CAUUCAGAAAGCUUCAAAU, antisense AUUUGAAGCUUUCUGAAUG and) were designed and commercially obtained along with a non-target scrambled one (SC). Around 0.3x106 cells were seeded in 6-well plates and incubated for 16–18 hrs. At 60–70% confluence, siRNA transfection was done using lipofectamine 3000 (L3000-008, Life Technologies) as per the manufacturer’s instructions. Briefly, 50 nM of PP1γ2-specific siRNAs and scrambled siRNA were diluted in Opti-MEM medium (11058-021, Life Technologies). Lipofectamine 3000 was also diluted in Opti-MEM medium accordingly. The siRNA-lipid complex (lipoplex) was prepared by mixing the diluted siRNA and lipofectamine 3000 and added to the cells. Following incubation for 12 hrs at 37°C in 5% CO2, exhausted media was replaced with a complete fresh medium.
Statistical analysis
The statistical analysis of the study was performed using the GraphPad Prism software (GraphPad, Version 5.00). ImaageJ (NIH) software was used for the densitometry analysis. Student’s t-test for comparisons between 2 groups and one-way analysis of variance for multiple groups of data were used for the evaluation of statistical significance. Differences were considered statistically significant at a p value < 0.05, and results were represented as mean ± SEM.
Results
Expression of PP1γ2 in cervical cancer HeLa cells
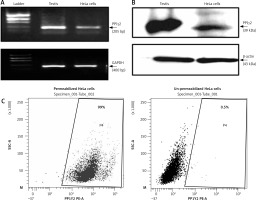
Expression of several germ cell-specific antigens have been reported in cancerous cells [16, 17], which can be explored as cancer biomarkers. Hence the expression of a testis-specific isoform of phosphatase PP1γ2 was explored in cervical cancer HeLa cells. Transcript expression of PP1γ2 was confirmed through RT-PCR, which showed an amplicon of ~205 bp specific for PP1γ2 in HeLa cells. Similar results were shown by human testis mRNA, which was used as a positive control, and GAPDH amplification confirmed the integrity of mRNA (Fig. 1 A). A further signal of ~39 kDa, specific for PP1γ2, was observed in HeLa cells as well as in the testis lysates in the western blot analysis (Fig. 1 B). Flow cytometry analysis of permeabilized HeLa cells also showed abundant intracellular expression of PP1γ2 (Fig. 1 C), whereas no signal was observed in un-permeabilized cells.
Fig. 1
Expression of protein phosphatases 1 gamma 2 (PP1γ2) in cervical cancer HeLa cells. A – HeLa cells showed PP1γ2 transcript (~205 bp) through RT-PCR; testis extract was used as control for PP1γ2 while glyceraldehyde 3-phosphate dehydrogenase (~400 bp) was used as an internal control, B – PP1γ2 protein (~39 kDa) expression was confirmed through western blot whereas β-actin (~43 kDa) was used as internal loading control, C – flow cytometry analysis of permeabilized live HeLa cells showed intense fluorescence of PP1γ2, whereas un-permeabilized cells did not show any fluorescence indicating predominant intracellular expression of PP1γ2
Localization profile of PP1g2 in cervical cancer HeLa cells
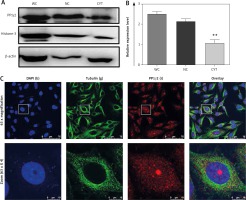
Western blotting confirmed the abundant expression of PP1γ2 in the cells’ nuclear fraction, while cytoplasmic fraction showed a comparatively lower expression profile (Fig 2 A). The intensity of the whole cell lysate was higher compared to both the fractions through densitometry analysis (Fig 2 B). Expressions of β-actin and Histone-3 served as controls for the cytoplasmic and nuclear fraction, respectively. Indirect immunofluorescence through confocal microscopy also localized PP1γ2 predominantly in the HeLa cells’ nuclear region, whereas discrete patches were observed in the cytoplasm. However, tubulin was found to be localized in the cytoplasm (Fig. 2 C).
Fig. 2
Differential expression of protein phosphatases 1 gamma 2 (PP1γ2) in whole cell (WC), nuclear (NC) and cytosolic (CYT) fractions of cervical cancer HeLa cell. A – western blot showing PP1γ2 protein expression in the WC, NC and CYT fractions of HeLa cells, B – densitometry analysis of the western blot confirmed the differential expression of PP1γ2. β-actin and Histone-3 were used as a loading control for the cytoplasmic and nuclear fractions, respectively. These results are presented as the mean ± SEM. of 3 independent experiments, **p < 0.01; as compared to WC, C – sub-cellular localization of PP1γ2 in HeLa cells showed predominant expression of the PP1γ2 (r) in the nuclear region of the cell, while discrete patches were also observed in the cytoplasm. Blue (b) represents nuclear stain DAPI and green (g) staining shows tubulin expression. Zoom represents 4x magnification of the original. All images were captured using an oil immersion 63x objective lens
Modulation of PP1γ2 during mitotic transition
To understand the modulation of PP1γ2 in cell cycle regulation, localization of PP1γ2 along with tubulin was evaluated in the various stages of dividing HeLa cells. PP1γ2 was localized to the nucleus of the mononuclear cells, whereas signal of tubulin was noticed in the cytosol (Fig. 3 A-i). At the initiation of the cell division, PP1γ2 redistributed to the poles, whereas tubulin was localized to both the spindle poles and the cytosol (Fig. 3 A-ii). In the mitotic phase, PP1γ2 was found to be condensed and merged entirely with tubulin to the poles of the bipolar cells (Fig. 3 A-iii). After complete cell division, PP1γ2 re-localized again back to the nucleus even before the completion of cytokinesis; however, tubulin remained in the cytosol only (Fig. 3 A-iv) and did not overlay with PP1γ2.
Fig. 3
Spatio-temporal distribution of protein phosphatases 1 gamma 2 (PP1γ2) (Red) and tubulin (green) in HeLa cells. A – in dividing HeLa cells: PP1γ2 is localized to the nucleus of the mononuclear cells, whereas tubulin localized in the cytosol (I). PP1γ2 started redistributing to the poles at the initiation of the cell division, where tubulin localized to both the spindle poles as well as to the cell periphery (II). Localization of PP1γ2 merged completely with tubulin to the poles of the bipolar cells in mitotic phase (III). After complete cell division, PP1γ2 re-localized again back to the nucleus, whereas tubulin remained in the cytosol only (IV), B – in Taxol-induced multipolar HeLa cells, increased expression of PP1γ2 merged completely with tubulin on multiple spindle poles of all the multipolar cells, including tripolar (I), tetrapolar (II), and pentapolar cells (III). Punctuated staining of PP1γ2 was observed in the vicinity of the distorted chromosomal apparatus, where tubulin remained located in the cytosol itself (IV). Densitometry analysis through ImageJ software showing relative expression of PP1γ2 in (C) single dividing HeLa cells, D – in Taxol-induced multipolar HeLa cells
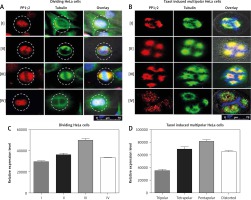
Furthermore, to comprehend the dynamics of PP1γ2 during cell division, multinuclear cells were induced with Taxol. In the Taxol-induced multipolar cells, elevated expression of PP1γ2 merged completely with tubulin on multiple spindle poles, including tripolar (Fig. 3 B-i), tetrapolar (Fig. 3 B-ii), and pentapolar cells (Fig. 3 B-iii). Punctate staining of PP1γ2 was observed in the distorted chromosomal apparatus’s vicinity, whereas tubulin remained located in the cytosol itself (Fig. 3 B-iv). These observations clearly indicate that PP1γ2 has a specific pattern of modulation during the progression of the cell cycle.
PP1γ2 is upregulated during hypoxia in cervical cancer HeLa cells
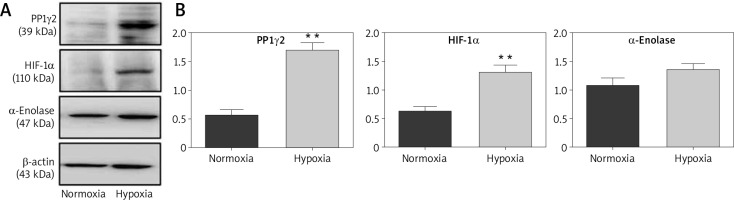
Hypoxic conditions are known to be favourable for cancerous cells, but the exact rationale to make this environment favourable for cancerous cells remains unclear. Therefore, HeLa cells were grown under a hypoxic environment (1% oxygen) and assessed for the expression of PP1γ2 through western blotting. Cells grown under a standard environment (normoxia, 21% oxygen) were used as control. Interestingly, the results showed up-regulation of PP1γ2 in hypoxic conditions, where upregulated levels of HIF1-α and α-Enolases were used as the known markers of hypoxic conditions (Fig. 4 A). Densitometry analysis confirmed these results with statistical significance for PP1γ2 & HIF1-α, whereas upregulation of α-Enolase was not statistically significant (Fig. 4 B).
Fig. 4
Upregulated expression of protein phosphatases 1 gamma 2 (PP1γ2) in hypoxic condition of cervical cancer HeLa cells. A – western blots showing the upregulation of PP1γ2, HIF1-α, and α-enolase in hypoxic environment of HeLa cells, B – densitometry analysis confirmed these results with the statistical significance for PP1γ2 and HIF1-α, whereas upregulation of α-Enolase was not statistically significant. At least 3 independent experiments were done and data was normalized with β-actin, the results are presented as mean ± SEM **p < 0.01
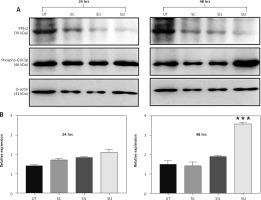
PP1γ2 silencing increases GSK3β phosphorylation
It has been proven before that GSK3β is a direct substrate for PP1γ2 and dephosphorylates pGSK3β (Ser 9) to produce an active form of GSK3β [18]. An experiment was designed to assess the phosphorylation level of GSK3β after the silencing of the expression of PP1γ2 through siRNA. Hence 2 siRNAs, including Si1 & Si2, were designed specifically to silence the expression of PP1γ2. Western blotting of these transfected cells revealed that siRNA-mediated silencing of PP1γ2 downregulated the expression of PP1γ2 and increased the phosphorylation of GSK3β [19, 20]. Densitometric analysis of the data further confirmed the increased phosphorylation of GSK3β within 24 hrs, which was significant within 48 hrs for Si2 treated cells (Fig. 5 A, B).
Fig. 5
Protein phosphatases 1 gamma 2 (PP1γ2) silencing increased the phosphorylation of GSK3β. A – PP1γ2-siRNA treatment in HeLa cells downregulated the expression of PP1γ2 (~39 kDa) and increased phosphorylation of GSK3β (~46 kDa), B – densitometric analysis of PP1γ2-silenced cells further confirmed increased phosphorylation of GSK3β within 24 hrs, which was quite significant within 48 hrs of Si-1 treatment. At least 3 independent experiments were done, and data of the study are presented as mean ± SEM, ***p < 0.001, compared to UT
Discussion
Various isoforms of PP play a pivotal role in cell cycle regulation [21] and cytokinesis [21] while being responsible for mitotic exit. Two isoforms of PP1γ are known to express by the alternate splicing of the PPP1CC gene. PP1γ1 is expressed ubiquitously and has been explored intricately in cell cycle regulation [10]; however, PP1γ2 as a testis-specific isoform [9] has not been investigated extensivley with respect to cell cycle regulation and tumourigenesis. The present study reports the expression of PP1γ2 RNA (Fig. 1 A) as well as protein (Fig. 1 B) in cervical cancer HeLa cells. Flow cytometry analysis revealed prominent subcellular expression of PP1γ2 in permeabilized HeLa cells, whereas no signal was observed in the unpermeabilized HeLa cells depicting no cell surface expression of PP1γ2 in HeLa cells (Fig. 1 C).
Earlier reports revealed enriched PP1 activity in the nucleus of the cell [22, 23]. To assess the subcellular distribution of PP1γ2 in HeLa cells, the protein was extracted from whole-cell, nuclear and cytoplasmic fractions and subjected to western blotting; these results demonstrated predominant expression of PP1γ2 in the nucleus as compared to the discrete distribution in the cytoplasmic fraction (Fig. 2 A, B). Furthermore, immunofluorescence studies also localized PP1γ2 to the nucleus of mononuclear interphase HeLa cells (Fig. 2 C) and relocated to the spindle poles during the mitotic phase. In the dividing cells, PP1γ2 redistributed to the spindle poles and appeared to be more condensed at the poles, completely merged with the tubulin localization. At the same time, as soon as cell division was completed, PP1γ2 localized back to the nucleus of the divided cells (Fig. 3 A), whereas tubulin localization was seen in the cytosol only. Hence, these observations can speculate that PP1γ2 may interact with the spindle organizing apparatus transiently and may regulate the tubulin for spindle pole formation.
To confirm the spatio-temporal redistribution of PP1γ2 in dividing cells, multipolar HeLa cells were induced by Taxol. As Taxol inhibits tubulin depolymerization and arrests the cells in the G2/M phase, and accelerates apoptosis [6], these cells showed intense staining of PP1γ2 at the multiple spindle poles (Fig. 3 B) and overlaid completely with the tubulin staining. Because these multipolar cells become polyploid and eventually go through apoptosis, intense localization of PP1γ2 on the poles indicates its possible role in regulating spindle formation and cell division.
To understand the effect of the hypoxic tumour microenvironment on PP1γ2, its expression level was observed under hypoxic conditions. Hypoxia is a prominent characteristic of cancerous cells governed by hypoxia-inducible factor-1α (HIF-1α), a key transcription factor, and regulates many downstream targets [24]. In cancerous cells, the hypoxic condition has been associated with radio- and chemoresistance and inhibits apoptosis, which alters EMT cell signalling and genomic stability and induces downstream cell proliferation. Observations and findings from the present data demonstrated an upregulated level of PP1γ2 (Fig. 4). Hence, it might be associated with a defensive role against hypoxia-induced stress, which promotes cancer cell survival under hypoxic conditions and thus leads to poor prognosis.
Further to assess the functional role of PP1γ2 with downstream effectors, siRNA-mediated silencing of PP1γ2 in HeLa cells showed increased phosphorylation of GSK3β (Fig. 5). The role of GSK3β has been reflected in the cell cycle regulation of cancerous cells[18]. Simultaneously, the interaction of PP1γ2 with GSK3β has been reported in spermatocytes [25], where it regulates spermatogenesis. GSK-3β is well documented to participate in a complex array of critical cellular processes. During mitosis, a phosphorylated form of the GSK-3β (p-GSK-3β) accumulates at centrosomes and spindle poles. GSK-3β is a direct substrate for PP1γ2; where GSK-3β is being dephosphorylated through PP1γ2 and produces an active form of GSK-3β. Earlier studies have also predicted the role of active GSK-3β (dephosphorylated form) in the regulation of microtubule dynamics [26].
In the present study, silencing of PP1γ2 made it unavailable to dephosphorylate GSK-3β and led to the increased phosphorylated form of the GSK-3β (p-GSK-3β); at the same time, total GSK-3β did not show much difference. Interestingly, the silencing of PP1γ2 through siRNA upregulates the phosphorylation of GSK3β and hence also affects the downstream signalling. Therefore, silencing of PP1γ2 may indirectly target GSK3β and it remains inactive in the phosphorylated form, which regulates the downstream microtubule dynamics.
Conclusions
Tumour cells frequently express genes that are normally restricted to the testis, known as cancer/testis antigens. Similarly, so far, expression of PP1γ2 had been reported as a testis-specific isoform; however, for the first time, the present study demonstrated a spatio-temporal redistribution of PP1γ2 during the cell cycle of HeLa cells. Silencing of PP1γ2 through siRNA also demonstrated the regulation of GSK3β phosphorylation, which is a well-known regulator of the cell cycle; hence this study verified PP1γ2 as an upstream player, where PP1γ2 modulation plays an important role during cell cycle regulation.








