Introduction
Hepatocellular carcinoma (HCC) ranks number three of cancer-related deaths [1]. The aging population and improvements in medical technology have contributed to an increase in the prevalence of multiple primary malignant neoplasms (MPMNs) [2]. Warren and Gates studied the condition of multiple primary malignant cancers and established some diagnostic criteria in 1932; over 1200 case reports were analyzed. These criteria are still being accepted at present [3]. Multiple primary malignancies (MPMs) in a single patient are rare. In reviews of the literature regarding multiple primary malignancies, the overall incidence is between 0.73% and 11.7% [4]. The multi-detector computed tomography results of 74 individuals with synchronous HCC and other solid tumors were reviewed in this retrospective analysis.
Material and methods
Patients
The institutional research ethics review committee has approved the study, and informed consent from the patient was waived due to the retrospective design of this study. This retrospective study included 74 synchronous HCC and other solid malignancies between April 2007 and August 2020. They were 41 women and 33 men (mean age, 63.36 years; range, 50-78 years) (Table 1). All the 74 patients had ultrasonography. Forty-three patients were positive for hepatitis C virus. Nine patients were positive for the hepatitis B virus. Twenty-two patients were positive for hepatitis C and B viruses (Table 2). The pathological diagnoses of all 148 malignancies were confirmed in all 74 cases. All of the patients had laboratory abnormalities. On admission, these aberrations revealed the following: slightly increased total bilirubin 1.4-4 mg/dl (normal 0.1-1.1), aspartate aminotransferase (AST) 50-160 IU/ml (up to 40) and alanine aminotransferase (ALT) 50-140 IU/l (up to 40). In 55 cases, the alpha-fetoprotein (AFP) titer was more than 300 ng/ml (normal range 0-10 ng/ml). All the patients showed radiological signs of cirrhosis. Although characteristic computed tomography (CT) findings of HCC were detected in all 74 patients, fine-needle aspiration cytology (FNAC) was done to exclude the possibility of metastases and confirm the histopathological diagnosis. Except for three individuals, all HCCs and other synchronous malignancies were detected simultaneously.
Table 1
Sex characteristics of our 74 patients
| Sex | Frequency | Percent | Valid percent | Cumulative percent |
|---|---|---|---|---|
| Male | 33 | 44.6 | 44.6 | 44.6 |
| Female | 41 | 55.4 | 55.4 | 100.0 |
| Total | 74 | 100.0 | 100.0 |
Table 2
Association of chronic hepatitis virus infection with synchronous hepatocellular carcinoma and other solid malignancies in 74 patients
| Parameter | Frequency | Percent | Valid percent | Cumulative percent |
|---|---|---|---|---|
| HCV | 43 | 58.1 | 58.1 | 58.1 |
| HCV & HBV | 22 | 29.7 | 29.7 | 87.8 |
| HBV | 9 | 12.2 | 12.2 | 100.0 |
| Total | 74 | 100.0 | 100.0 |
One patient had a history of breast cancer for five years with a history of HCC for one year. During follow-up of HCC after trans-arterial chemoembolization, there was incomplete ablation of HCC with newly developed multiple pulmonary nodules. FNAC was taken from a pulmonary nodule that revealed metastatic breast carcinoma. Biopsy from the pulmonary nodule underwent immunohistochemical staining. It was positive for mammaglobin, estrogen, and progesterone receptors. It was harmful to HepPar1 and glypican-3. These findings confirmed breast origin. The second patient had a history of radical mastectomy five months ago and underwent a follow-up CT scan that revealed multiple hepatic focal lesions characteristic of HCC. FNAC from a hepatic focal lesion revealed HCC. Finally, the third patient presented with ovarian carcinoma first.
Two and half months after radical hysterectomy for ovarian carcinoma, she showed a newly developed hepatic focal lesion that pathologically proved to be HCC. The Warren and Gates criteria for two primary tumors are now widely recognized [3]. The presence of two malignant locations confirmed histopathologically and with different histopathology in the two sites are the inclusion criteria of the patients in this study. Patient exclusion criteria included those whose second tumor was thought to be a metastasis of the first tumor and those whose tumors had not yet been histopathologically confirmed as distinct entities. A variety of information, including the patient’s age at diagnosis, sex, location of origin, histopathology, and clinical stage, has been documented. In the treatment of HCC, the Barcelona Clinic Liver Cancer (BCLC) staging system is the most often utilized [5]. So, it is our study’s staging system.
CT technique
For each of the 74 patients a 128 MDCT scanner (Philips Ingenuity; Philips Healthcare, Best, The Netherlands) was used to conduct whole body and triphasic abdominal CT scans. Pre-contrast and post-contrast images were taken with a slice thickness of 3 mm for both sets. Abdominal examinations were performed in both arterial and delayed phases. The portal phase was completed for the whole body. We used 120 ml of low osmolar nonionic contrast medium (Optiray 350, Ioversol) at a 4-5 ml/s flow rate for the post-contrast study. Throughout the whole process, patients were instructed to hold their breath. By automating the bolus monitoring and detection process, the precise timing of the early arterial phase may be achieved. The portal venous phase was conducted 55-60 seconds following the commencement of the contrast material infusion. The delayed phase was carried out with a delay of between three and six minutes. A workstation (Extended Brilliance Workspace V3.5.0.2254) (EBW) was then used to post-process all of the images. The images were viewed on lung, soft tissue, and bone windows. Twenty patients underwent an MRI examination for confirmation of CT findings.
Interpretation of images
Pre-contrast attenuation of lesions, density in all stages (arterial, portal, and delayed phases), number of lesions, vascular invasion, lymph node involvement, and other abdominal organs, as well as metastatic dissemination, were the primary focus of data interpretation and image analysis. The whole body’s lymph nodes and organs, as well as any bone or lung metastases, were evaluated using a CT scanner. All HCCs and other malignancies were assessed for local, lymphatic, hematogenous, or transcoelomic spread if suspected according to the primary site of the tumor. Cancer size, organ of origin, internal architecture, tissue invasions, vascular encasement, calcifications, and metastases were all evaluated. All 148 cancers were assessed for their tumor stage.
Results
Our research used the Warren and Gates criteria to determine extra-hepatic primary malignancies (EHPM) [3]. Within the 3480 individuals with hepatocellular carcinoma, the inclusion criteria for synchronous EHPM were satisfied by 74 patients (2.1%). Each of the 148 cancers was correctly identified, assessed, and staged. Hepatocellular carcinoma was identified in all 74 individuals. The most frequent extra-hepatic primary sites were breast (18/74, 24.3%) (Fig. 1), followed by kidney (15/74, 20.3%) (Fig. 2), lymphoma (9/74, 12.2%) (Fig. 3), uterus (7/74, 9.5%) [endometrium (4), myometrium (1) and uterine cervix (2)], ovary (5/74, 6.8%), colon (5/74, 6.8%), prostate (5/74, 6.8%), urinary bladder (3/74, 4.1%), thyroid (2/74, 2.7%), gallbladder (1/74, 1.4%), stomach (1/74, 1.4%), pancreas (1/74, 1.4%), esophagus (1/74, 1.4%) and lung (1/74, 1.4%) (Table 3). The HCC was classified in the BCLC staging system as stage A (40 individuals), B (24 individuals), and C (10 individuals) (Table 4). Breast cancer staging was stage IA (5 individuals), IIA (6 individuals), IIIB (3 individuals), and IV (4 individuals). Individuals diagnosed with renal cell carcinoma were stage I (12 individuals) and stage II (3 individuals). Non-Hodgkin lymphoma, diffuse large B-cell type, was found in all nine individuals. Ann Arbor’s staging of lymphoma was stage I (one individual), IE (2 individuals), II (3 individuals), IIE (one individual), and III (2 individuals). The International Federation of Gynecology and Obstetrics (FIGO) staging of ovarian carcinoma was stage IIC (3 individuals), stage III (one individual), and local recurrent (one individual). FIGO stagings of uterine malignancies were stage IB endometrial carcinoma (4 individuals), stage IB uterine sarcoma (one individual), and stage IIB cervical carcinoma (2 individuals); one of them showed regional malignant lymphadenopathy. Patients with colonic carcinoma were stage IIC (3 individuals), stage IIIB (one individual), and stage IIIC (one individual). Patients with prostatic carcinoma were stage II (3 individuals), stage III (one individual), and stage IV (one individual). One patient with urinary bladder carcinoma was stage III, and two patients were stage IV. Two patients with thyroid carcinoma were stage III. One patient with gall bladder adenocarcinoma was stage IVB. One patient with gastric carcinoma was stage IIA. One patient with pancreatic carcinoma was stage IIA. One individual with esophageal carcinoma was stage Ib. One individual with bronchogenic carcinoma was stage IIIA.
Fig. 1
64-year-old female patient with a history of chronic liver disease presented with skin edema of left breast. A, B) Portal phase demonstrates left breast mass (arrow) with thickened of related skin. C) Arterial, D) portal and E) delayed phases demonstrate segment IV hepatic exophytic focal lesion (enhanced in arterial phase, washout in portal and delayed phases (characteristic of HCC) (arrow))
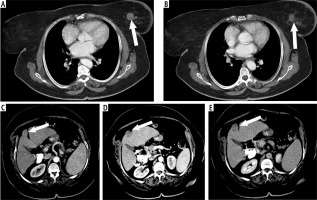
Fig. 2
58-year-old male patient with history of chronic liver disease presented with hematuria. A, B) Arterial phase demonstrates enhanced multiple hepatic focal lesions (arrow). C, D) Right renal mass (asterisk) enhanced in arterial phase (C) and washout in portal phase (pathologically proven renal cell carcinoma). Note regional renal hilar lymphadenopathy. E, F) Delayed phase demonstrates washout of hepatic focal lesions (characteristic of multifocal HCCs) (arrow)
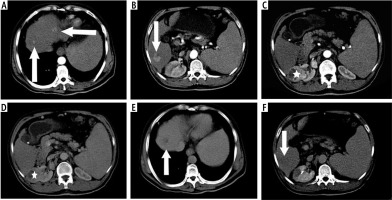
Fig. 3
62-year-old male patient with history of chronic liver disease presented oropharyngeal soft tissue mass. A) Arterial phase demonstrates enhanced hepatic focal lesion, and B) the delayed phase demonstrates washout out (characteristic of HCC) (arrow). C) The portal phase demonstrates soft tissue mass at the right side of the oropharynx (pathologically proven NHL) (asterisk)
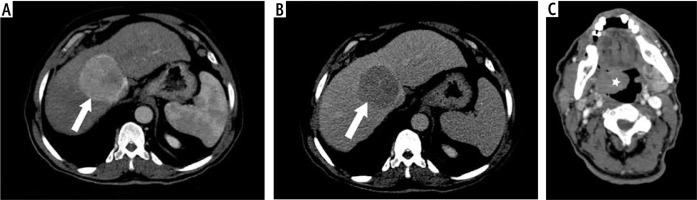
Table 3
Distribution of extra-hepatic synchronous primary tumor in 74 patients with hepatocellular carcinoma
Discussion
Synchronous primary multiple malignancies are tumors that occur simultaneously or within six months after the development of the initial cancer [6]. Warren and Gates [3] outlined the definition of a patient with dual primary cancers. For each tumor to meet these conditions, it must show clear evidence of malignancy, be distinct from the others, and have the potential of becoming a metastasis of the other tumor ruled out as plausible. Multiple primary cancers have steadily increased during the last decade [7].
If a patient is diagnosed with synchronous HCC with other solid malignancies, a biopsy from each tumor is still mandatory to identify the underlying tumor’s histological origin and choose the appropriate treatment plan [8]. The histopathology of each of the 148 cancers in our research was examined.
Having a second primary malignancy at an older age is unquestionably more likely [7], in line with our findings, which showed that the average age was 63.6 years.
HCC, as a prevalent malignancy in Asia and Africa, has also revealed a notable rise in the number of cases and deaths throughout North America and Europe [9].HCC mainly presents with underlying cirrhosis in more than 90% of cases [10]. The risk factors for HCC include cirrhosis, hepatitis C, and hepatitis B [11]. All the cases in our study show signs of cirrhosis. Fortythree individuals were positive for hepatitis C virus. Nine patients were positive for the hepatitis B virus. Hepatitis C and B viruses were found in 22 people.
In the context of HCC, a second primary tumor is rather prevalent, with clustering of genitourinary and gastrointestinal cancers [11, 12]. The incidences of intra-abdominal malignancies such as renal, hepatic, and pancreatic cancer were more significant in the synchronous group than in other groups. Most synchronous cancers were found in the abdominal cavity during the preoperative workup [13]. Our study found that 50/74 patients with abdominal malignancies had synchronous extra-hepatic original malignancies. These 50 cancers were renal cell carcinoma (15), abdominal lymphoma (7), ovarian carcinoma (5), endometrial carcinoma (4), myometrial sarcoma (1), uterine cervical carcinoma (2), colonic carcinoma (5), prostatic carcinoma (5), urinary bladder carcinoma (3), pancreatic carcinoma (1), gallbladder carcinoma (1), and gastric carcinoma (1).
Liver and HCC imaging has long relied on CT for initial tumor definition and post-treatment follow-up to evaluate response to therapy [14]. Using MDCT is an effective way to assess synchronous primary solid double malignancies [15-23]. This is in line with our findings, which show that MDCT correctly identified all 148 cancers. The radiological findings of HCC in the MPMs were the same as those of HCC alone patients. This agrees with our results as characteristic CT findings were detected in all 74 HCCs. Triphasic CT may aid in identifying hepatic nodules by providing helpful information about their possible causes [24].
There is variation in the incidence of the second EHPM site in individuals with HCC based on the study’s geographic dispersion [11]. The most prevalent locations in the Western series are the colorectal and genitourinary (mainly prostate) cancers [25]. In previous Japanese investigations, the most common EHPM in HCC patients was stomach cancer [26, 27]. Hong et al. [28] found that colorectal cancer is the most prevalent location of EHPM, followed by stomach cancer, breast cancer, and kidney cancer. Colorectal carcinoma (5 cases), head and neck carcinoma (4 cases), genitourinary cancer (3 cases), lymphoproliferative malignancies (2 cases), bronchogenic carcinoma (1 case), skin melanoma (1 case), breast carcinoma (1 case), and 2 malignancies of unknown primary origin were found to be the most frequently associated malignancies in another study [29]. According to our findings, breast cancer is the most prevalent non-hepatic malignancy (18/74). According to previous research, the hepatitis virus has been linked to an elevated risk of breast cancer [30-32]. The second most common extra-hepatic malignancy is renal cell carcinoma (15/74). The third most common extra-hepatic malignancy is lymphoma (9/74). This may be due to the association of hepatocellular carcinoma and lymphoma with the hepatitis C virus, which agrees with previous studies [33, 34].
The association between chronic hepatitis viral infection and many different lymphoproliferative malignancies has been widely researched [33]. Di Stasi et al. [35] found that the second most common extra-hepatic neoplasm (28.6%) consisted of lymphoproliferative disorder of B-cell type, with a higher incidence than expected in the reference population. This difference may be due to the relatively small number of cases and the geographical setting. Our study shows 9 cases of lymphoma; 7 of them are positive for hepatitis C and B viruses, and 2 cases are positive for hepatitis C virus. However, this result does not agree with other series demonstrating a relatively low incidence of B-cell lymphoproliferative disorders in HCC individuals [11].
Limitations
Our study had some limitations. First, there was selection bias. Second, the sample size was not very large. Third, the follow-up period was not long. Fourth, our retrospective study includes data from a single hospital.
Conclusions
During pretreatment evaluation, the possibility of synchronous double solid malignancies with HCC should always be considered. MDCT scanning is a reliable imaging method for evaluating synchronous HCC and other solid tumors. New staging for a combination of synchronous HCC and other solid tumors may be added to accommodate new treatment methods. Immunological and genetic aspects of synchronous double malignancies must be evaluated. Examining people from diverse geographic and core regions would provide more compelling results.






