Myocardial ischaemic loss in acute myocardial infarction (AMI) remains the most significant contributor to chronic heart failure and sudden cardiac death [1]. Despite the progress in pharmacological and invasive management of AMI, the prevalence of ischaemic heart failure will grow substantially in the next decades [2]. Novel therapeutic approaches, including stimulation of cardiac repair and regeneration, are needed [2, 3]. Evidence in animal models [4] and humans [5–7] shows a modifiable balance between the irreversible and reversible myocardial injury in acute ischaemia (depending on factors such as the timing of revascularization and associated therapy), presenting a mechanistic room for novel therapeutic approaches.
In our recent pilot study, transcoronary administration of 30 × 106 standardized, multipotent Wharton jelly mesenchymal stem cells (WJMSC) 5 to 7 days after AMI was safe [5], and it was associated with minimal left ventricular remodelling and improved haemodynamics throughout 3 years of follow-up [8], providing the basis for a larger-scale clinical trial. Here, we present multi-modality imaging in the CIRCULATE-AMI pilot study cohort as a framework for an imaging endpoint-powered, randomized controlled trial of WJMSC use to stimulate myocardial repair/regeneration.
A 57-year-old man with hypertension and hypercholesterolaemia was admitted to the Jagiellonian University Department of Cardiac and Vascular Diseases, John Paul II Hospital in Krakow, Poland due to an acute anterior ST-segment elevation AMI as the first manifestation of coronary artery disease. The patient reported acute chest pain and dyspnoea that lasted 4 h before the first medical contact. Urgent coronary angiography showed an acute occlusion in the proximal segment of the left anterior descending artery (LAD) and a severe stenosis of the marginal branch (Figure 1 I A). Primary percutaneous coronary intervention (PCI) with stent implantation (XienceTM 3.5 mm, Abbott, USA) was performed, followed by a second stage PCI of the marginal branch (XienceTM 2.75 mm, Abbott, USA) 3 days later, resulting in complete coronary revascularization (Figure 1 I B). The peak hs-troponin T level was 4.28 ng/ml (ULN < 0.014 ng/ml), and CK-MB activity peaked at 272 U/l (ULN < 24 U/l), consistent with a major myocardial tissue loss. Maximized guideline-indicated pharmacotherapy was introduced for acute-phase AMI and post-AMI left ventricular (LV) dysfunction prevention.
Figure 1
A 57-year-old man was urgently admitted due to ST-segment elevation acute myocardial infarction. Left anterior descending artery occlusion (I A) was treated successfully with primary angioplasty with stent implantation; 3 days later, coronary revascularization was completed by stent-supported angioplasty of a severely narrowed marginal branch (I B). A blood level of cardiac myonecrosis markers was consistent with a major loss of the contractile tissue. The patient consented to participation in the CIRCULATE-AMI Pilot Study Cohort. Multi-modality myocardial imaging was performed involving echocardiography, perfusion single-photon emission computed tomography (SPECT) and gated SPECT (G-SPECT), and cardiac magnetic resonance imaging (cMRI) (II–V A, B). On day seven 30 × 106 Wharton’s jelly pluripotent stem cells (50% of these – labelled with 99mTc-sestamibi) were delivered to the infarct zone using the transcoronary route (infarct-related artery), and a perfusion catheter dedicated to transcoronary delivery of cell-based therapies (I C). Whole-body scintigraphy revealed an intense (31.8%) cell retention in the heart (delineated with a red line; note an only minor label uptake in the lungs [arrows], with a great majority of the label in the anterior wall and septum zone (I D). Two-chamber echocardiography performed at baseline showed akinetic left ventricle anterior wall (arrows) with severe ejection fraction impairment (36%; II A and II B). At 6 months after cell transfer the examination showed partial anterior wall contractility restoration (arrows) with an increase in LVEF to 51%. Myocardial perfusion (longitudinal view in III A, 2D-bullseye view in III B) showed a baseline severe perfusion defect in the antero-septal and apex regions of the left ventricle. At 6 months there was a marked improvement of perfusion in the anterior and septal region of left ventricle, with a relative reduction of the non-perfused zone by 22.6% (III D, III E). 3D dynamic visualization of LV contractility (G-SPECT) showed a baseline EF of 35% at rest and (IV A) with a stress increase to 41% (IV B). Both resting and stress LVEF markedly improved at 6 months (IV C and IV D). Two-chamber and 4-chamber cMRI performed at baseline showed infarcted areas (white arrows, late enhancement zones) with areas of complete no-flow (red arrows) (V A, V B). Baseline LVEF by cMRI was 37%. At 6 months, no-flow zones were no longer detected while the areas of late enhancement were significantly reduced (V C, V D). LVEF increased to 55%
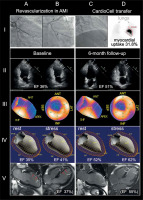
Imaging modalities performed in the AMI post-acute stage (day 5–6 to minimize the effect of stunning/neurogenic impairment [5]) included 2-dimensional echocardiography, TTE (Philips EPIQ 7), single-photon emission computed tomography, SPECT (GSQUAN, E.CAM, Siemens), and gadolinium-enhanced cardiac magnetic resonance imaging, cMRI (Siemens Magnetom Sonata 1.5T) (Figure 1). In a consistent manner, those showed septal and anterior wall akinesia and severely reduced left ventricular ejection fraction (LVEF respectively 36%, 35%, and 37%) together with an early adverse remodelling of the LV (Figure 1). CMRI large areas of late enhancement with microvascular obstruction, and perfusion defect in the anterior wall, septum, and apex (SPECT) were consistent with the extensive myocardial loss indicated by blood markers of cardiomyocyte necrosis.
On day 7 after AMI, 30 × 106 standardized mesenchymal stem cells (WJMSC; 50% cells labelled with 99mTc-exametazime) were delivered via the infarct-related artery using a novel catheter system dedicated to transcoronary administration of cell-based therapies [7] (Figure 1 I C). Cell label myocardial uptake by whole-body SPECT was 31.8% (Figure 1 I D), consistent with a large proportion of WJMSC cells homing to the infarct area.
The in-hospital and cardiac rehabilitation course was uneventful, and the patient – despite having suffered a major myocardial tissue loss – returned to their (office) work and daily activities.
Six months later, the clinical follow-up was unremarkable (absence of angina, absence of clinical heart failure). Repeated imaging (Figure 1 II–V C, D) showed an improvement in LVEF, with the absolute of +15% by TTE, +17% by SPECT, and +18% for MRI and a reduction in end-diastolic LV volumes (–19% TTE, –22% SPECT, –26% for MRI). The total perfusion deficit by quantitative perfusion scintigraphy decreased from 31% to 24% (absolute reduction by 7%, relative reduction by 22.6%). Clinical monitoring showed no mid-term adverse effects of WJMSC administration (including those potential or possible).
These findings support our further use of multimodal imaging of the evolution of left ventricular dysfunction, ischaemia, and remodelling in a randomized, controlled, double-blind clinical trial of standardized WJMSC use to stimulate myocardial repair and regeneration in acute myocardial infarction (NCT03404063).








