Summary
Inflammatory cell count may enable simple prediction of the location of calcified coronary lesions. In the regression analysis, we found a significant correlation between neutrophil count below 5.01 × 109/dl, lymphocyte count below 1.95 × 109/dl, and the proximal location of the culprit lesion. Based on our analysis, lower neutrophil and lymphocyte counts are more representative for proximal, while higher counts are more representative for non-proximal location of calcified coronary lesions, independently of the number of diseased vessels.
Introduction
Coronary artery disease (CAD) is still one of the leading causes of death in developed countries [1]. Risk factors include age, sex, arterial hypertension, hypercholesterolemia, diabetes mellitus, chronic kidney disease, obesity, and family history, but also physical activity and dietary habits [2, 3]. The clinical profile of the current cardiovascular population has changed, including patients with advanced diabetes, heart failure and multivessel disease. Severe calcifications in coronary arteries may significantly limit the efficacy of standard percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Both types of revascularizations had to evolve to improve short- and long-term outcomes. The 10-year results of the SYNTAX trial did not reveal significant differences in the all-cause mortality between percutaneous interventions (PCI) and CABG [4]. A pooled analysis of four randomized studies [5] by Gaba et al. proved that the relative advantages and disadvantages of PCI vs. CABG were consistent regardless of acute coronary artery status. The latest results of the SWEDEHEART registry showed that CABG in patients with left main disease was associated with lower mortality and fewer major adverse cardiovascular events compared to PCI [6]. Obstructive atherosclerotic lesions characterized by high calcium content may be treated by ablation techniques: rotational atherectomy (RA) [7], orbital atherectomy (OA), excimer laser coronary atherectomy (ELCA) and intravascular lithotripsy (IVL) [8].
Improvement in revascularization techniques requires a deep understanding of the pathophysiology of coronary artery disease and the mechanism which may influence the outcomes. The role of immune system activation in atherosclerotic plaque development and progression has been postulated [7, 9]. Activated neutrophils play a significant role in atherosclerosis development by various cytokines and damage-related molecules, and reactive oxygen species generation followed by phagocytosis [10]. The inflammatory-related severity of coronary artery disease was presented by Liu et al. [11]. However, less is known about the inflammatory cells’ representation-dependent calcification and lesion location in the coronary bed.
Aim
The aim of the study was to analyze simple features of the whole blood count in relation to the location of calcified atherosclerotic lesions in patients treated with coronary rotational atherectomy (RA).
Material and methods
Eighty-one consecutive patients who underwent RA were included in this analysis.
Patients with inflammatory, oncological or rheumatological diseases were excluded. Demographical and clinical history was collected. Blood samples were obtained at admission. Baseline blood cell counts were analyzed, and inflammatory indexes, including neutrophil to lymphocyte ratio (NLR), were calculated. Platelets’ and red blood cells’ basic analysis was also undertaken.
All patients were treated with optimal guideline-directed medical therapy. In 56 patients, stable chronic coronary syndrome and in 25, unstable angina was diagnosed. Patients with acute myocardial infarction were excluded from the analysis.
All RA procedures were performed by the same team of experienced operators after a heart-team discussion. Patients were referred for RA in case of the presence of massive calcifications preventing device delivery or full expansion of coronary balloons. RA procedures were performed in a standard way, using burrs with diameters of 1.25–1.75 mm at a speed of 140 000–180 000 RPM.
Upon visual analysis of coronary angiography, patients were divided into two subgroups with proximal (group 1) and non-proximal (group 2) culprit lesion location. The proximal location of the culprit lesion was defined according to previous definitions [12].
Multivessel disease was diagnosed if lesions of more than 50% diameter stenosis were observed in more than one major coronary artery or its branches. The lesion was considered a culprit if stenosis diameter was greater than 70%.
All the patients were treated according to chronic coronary syndromes guidelines available during the time of analysis, which included: antiplatelets, angiotensin-converting enzyme inhibitors, β-blockers and statins or statin/ezetimibe combination [13].
Periprocedural myocardial infarction was defined according to Fourth Universal Definition [14]. The study was performed as a retrospective analysis and was approved by the Institutional Bioethics Committee (no. 55/20).
Statistical analysis
Continuous variables were reported as medians and interquartile ranges (Q1–Q3) since data did not follow a normal distribution. Categorical data were presented as numbers and percentages. The comparison of interval parameters between proximal and non-proximal groups was performed by the Mann-Whitney test. Categorical data were compared by the χ2 test of independence. A logistic regression analysis was performed to identify potential predictors of proximal culprit location. Both univariate and multivariable models were used. The multivariable model was assessed by the best subset method. The results were presented as the odds ratio (OR) and its 95% confidence intervals (95%CI). Additionally, a receiver operating characteristic (ROC) curve was determined for the predictor score of the significant model in which neutrophil and lymphocyte counts separately and as combined parameters were incorporated as the predictors of proximal culprit location.
Results
Eighty-one consecutive patients (57 (70%) males, mean (SD) age of 70.4 ±8 years) underwent RA in our department after careful clinical assessment by the heart team. There were 74 (91%) patients with arterial hypertension, hypercholesterolemia was diagnosed in 69 (85%) and diabetes mellitus in 45 (56%) of them. Kidney dysfunction, defined as glomerular filtration rate (GFR) below 60 ml/min/1.73 m2, was found in 28 (35%) patients, and 14 (17%) suffered from peripheral artery disease.
The two subgroups did not differ significantly regarding demographic or clinical data, as presented in Table I.
Table I
Demographic and clinical data presenting differences between patients with proximal (group 1) and non-proximal (group 2) atherosclerotic plaque location in coronary arteries
| Parameter | Group 1 n = 43 | Group 2 n = 38 | P-value |
|---|---|---|---|
| Demographic: | |||
| Age [years] median (Q1–Q3) | 70 (65–78) | 70 (66–74) | 0.51 |
| Sex (m/f) (%) median (Q1–Q3) | 21/22 | 22/16 | 0.50 |
| Weight [kg] median (Q1–Q3) | 83 (74–95) | 87 (80–94) | 0.42 |
| Height [kg] median (Q1–Q3) | 171 (161–176) | 168 (162–176) | 0.20 |
| Clinical – coronary: | |||
| Acute coronary syndrome (%) | 21 (49) | 22 (58) | 0.50 |
| Clinical – co-morbidities: | |||
| Arterial hypertension (%) | 26 (61) | 29 (76) | 0.11 |
| Hypercholesterolemia (%) | 28 (65) | 22 (58) | 0.14 |
| Diabetes mellitus (%) | 29 (67) | 26 (68) | 0.32 |
| Nicotine – current smoker (%) | 10 (23) | 5 (13) | 0.11 |
| Nicotine in past (%) | 3 (0.7) | 1 (0.3) | 0.28 |
| COPD (%) | 7 (16) | 7 (18) | 0.91 |
| PAD (%) | 10 (23) | 12 (32) | 0.74 |
| Kidney dysfunction* (%) | 12 (28) | 10 (26) | 0.74 |
| AF (%) | 8 (21) | 10 (26) | 0.52 |
| Stroke (%) | 1 (0.2) | 4 (11) | 0.19 |
| Post: | |||
| Myocardial infarction (%) | 14 (33) | 10 (26) | 0.21 |
| Post – PCI (%) | 16 (37) | 15 (40) | 0.51 |
| After CABG (%) | 10 (23) | 5 (13) | 0.11 |
| Laboratory results: | |||
| WBC [× 109/dl] median (Q1–Q3) | 6.5 (5.89–7.40) | 8.47 (7.29–9.81) | < 0.001 |
| Neutrophils [× 109/dl] median (Q1–Q3) | 4.33 (3.86–5.31) | 5.58 (4.48–7.05) | 0.004 |
| Lymphocytes [× 109/dl] median (Q1–Q3) | 1.38 (1.13–1.66) | 1.87 (1.37–2.24) | 0.006 |
| Monocytes [× 109/dl] median (Q1–Q3) | 0.37 (0.32–0.56) | 0.53 (0.42–0.68) | < 0.001 |
| NLR median (Q1–Q3) | 3.29 (2.55–4.52) | 3.38 (2.15–4.34) | 0.68 |
| Luc cells median (Q1–Q3) | 0.16 (0.01–0.20) | 0.20 (0.13–0.23) | 0.08 |
| RBC [× 109/l] median (Q1–Q3) | 4.58 (4.00–4.96) | 4.76 (4.27–4.92) | 0.30 |
| Hb [mmol/l] median (Q1–Q3) | 8.4 (7.7–9.3) | 8.70 (8.10–9.20) | 0.45 |
| Hct [%] median (Q1–Q3) | 40 (37–43) | 41 (37–44) | 0.33 |
| Platelets [× 109/dl] median (Q1–Q3) | 205 (171–258) | 225 (202–284) | 0.30 |
| RDW [%] median (Q1–Q3) | 13.8 (13.2–15.4) | 13.80 (13.00–14.70) | 0.91 |
| MCHC [× 109/dl] median (Q1–Q3) | 21.13 (20.86–21.65) | 21.32 (20.95–21.75) | 0.47 |
| MPV [× 109/dl] median (Q1–Q3) | 8.2 (7.1–9.2) | 8.05 (7.10–9.60) | 0.17 |
AF – atrial fibrillation, CABG – coronary artery bypass grafting, COPD – chronic obstructive pulmonary disease, f – female, Hct – hematocrit, m – male, MCHC – mean corpuscular hemoglobin concentration, MPV – mean platelet volume, NLR – neutrophil to lymphocyte ratio, PAD – peripheral artery disease, PCI – percutaneous intervention, Q – quartiles, RDW – red cell distribution width, RBC – red blood count, WBC – white blood count.
There was a significant difference between groups regarding white blood cell (p < 0.001), neutrophil (p = 0.004) and lymphocyte (p = 0.006) count in whole blood analysis.
The univariable analysis revealed the significance of lower white blood cell (OR = 0.70, 95% CI: 0.55–0.90, p = 0.004), neutrophil (OR = 0.76, 95% CI: 0.59–0.99, p = 0.040), and lymphocyte count (OR = 0.28, 95% CI: 0.11–0.70, p = 0.006) in the prediction of the proximal location of the culprit lesion. The multivariable analysis showed a similar correlation for neutrophil (OR = 0.75, 95% CI: 0.58–0.97, p = 0.030) and lymphocyte count (OR = 0.27, 95% CI: 0.11–0.68, p = 0.005), as presented in Table II.
Table II
Uni- and multivariable regression analysis for prediction of proximal atherosclerotic location in coronary arteries
| Parameter | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Chronic coronary syndrome | 0.84 | 0.33–2.18 | 0.73 | – | – | – |
| Demographic: | ||||||
| Sex | 0.58 | 0.22–1.54 | 0.27 | – | – | – |
| Age | 1.02 | 0.96–1.07 | 0.57 | – | – | – |
| Weight | 0.99 | 0.96–1.02 | 0.42 | – | – | – |
| Height | 0.99 | 0.97–1.01 | 0.47 | – | – | – |
| Clinical: | ||||||
| Arterial hypertension | 0.17 | 0.02–1.45 | 0.11 | – | – | – |
| Diabetes mellitus | 0.68 | 0.28–1.65 | 0.40 | – | – | – |
| Hypercholesterolemia | 2.60 | 0.72–9.46 | 0.15 | – | – | – |
| COPD | 0.41 | 0.72–2.40 | 0.33 | – | – | – |
| PAD | 0.86 | 0.27–2.73 | 0.80 | – | – | – |
| Nicotine | 0.32 | 0.10–1.04 | 0.06 | – | – | – |
| Kidney failure* | 0.83 | 0.33–2.07 | 0.69 | – | – | – |
| Atrial fibrillation | 0.71 | 0.26–1.94 | 0.50 | – | ||
| Stroke | 7.19 | 0.84–61.5 | 0.07 | – | ||
| Laboratory: | ||||||
| WBC | 0.70 | 0.55–0.90 | 0.004 | – | – | – |
| Neutrophils | 0.76 | 0.59–0.99 | 0.040 | 0.75 | 0.58–0.97 | 0.030 |
| Lymphocytes | 0.28 | 0.11–0.70 | 0.006 | 0.27 | 0.11–0.68 | 0.005 |
| Monocytes | 0.28 | 0.04–2.12 | 0.22 | – | – | – |
| NLR | 1.00 | 0.79–1.26 | 0.08 | – | – | – |
| LUC | 0.11 | 0.00–7.69 | 0.31 | – | – | – |
| RBC | 1.02 | 0.95–1.09 | 0.60 | – | – | – |
| Hb | 0.80 | 0.54–1.19 | 0.28 | – | – | – |
| Hct | 0.95 | 0.87–1.04 | 0.25 | – | – | – |
| Platelets | 1.00 | 0.99–1.00 | 0.47 | – | – | – |
| RDW | 0.99 | 0.75–1.31 | 1.00 | – | – | – |
| MCHC | 1.02 | 0.51–2.06 | 0.95 | – | – | – |
| MPV | 0.99 | 0.96–1.03 | 0.66 | – | – | – |
AF – atrial fibrillation, CABG – coronary artery bypass grafting, COPD – chronic obstructive pulmonary disease, f – female, Hct – hematocrit, m – male, MCHC – mean corpuscular hemoglobin concentration, MPV – mean platelet volume, NLR – neutrophil to lymphocyte ratio, PAD – peripheral artery disease, PCI – percutaneous intervention, Q – quartiles, RDW – red cell distribution width, RBC – red blood count, WBC – white blood count.
Chronic coronary syndrome vs. unstable angina
The Mann-Whitney tests revealed significant differences between chronic coronary syndrome and unstable angina patients regarding monocyte count (p = 0.004) and large unstained cells (p = 0.028). The regression analysis revealed that the clinical presentation (stable or unstable angina) was not a significant predictor of culprit lesion location (OR = 0.84, 95% CI: 0.33–2.18, p = 0.726).
Single vs. multivessel disease
The Pearson χ2 test showed that patients with multivessel disease differed significantly from those with single-vessel disease regarding sex (p = 0.037) and hypercholesterolemia (p = 0.016). The multivariable regression analysis for two (OR = 0.59, 95% CI: 0.21–1.67, p = 0.317) and three-vessel disease (OR = 0.53, 95% CI: 0.17–1.59, p = 0.256) did not reveal any significant correlation regarding the prediction of culprit lesion location.
Receiver operator curve analysis
The receiver operator curves were performed separately for lymphocytes, neutrophils and as a combined value.
Lymphocytes
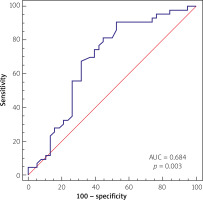
The multivariate analysis and ROC analysis revealed the predictive value of the low lymphocyte count for the prediction of proximal culprit lesion location, which presented an optimal cut-off value below 1.95 × 109/dl, yielding a sensitivity of 90.7% and specificity of 47.37% and area under the curve (AUC) of 0.684 (p = 0.003), as presented in Figure 1.
Neutrophils
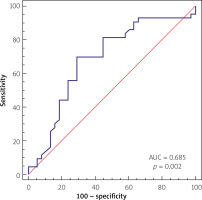
The multivariate analysis and ROC analysis revealed the predictive value of a low neutrophil count for the prediction of proximal culprit lesion location, which presented the optimal cut-off value below 5.01 × 109/dl, yielding a sensitivity of 69.77% and specificity of 71.05%, and the area under the curve of 0.685 (p = 0.002), as presented in Figure 2.
Combined analysis for neutrophil and lymphocyte count
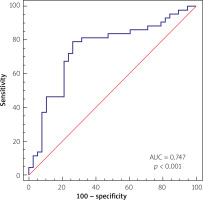
The multivariate analysis and ROC analysis revealed the predictive value of combined low neutrophil and lymphocyte count for the best prediction of proximal culprit lesion location, with the area under the curve of 0.747 (p < 0.001) yielding a sensitivity of 79.07% and specificity of 73.68%, as presented in Figure 3.
In-hospital outcomes
The in-hospital clinical course did not differ between the two groups. There were 3 (3.7%) periprocedural deaths (all patients in group 2). Two patients (1 in group 1 and 1 in group 2) experienced a periprocedural myocardial infarction (2.5%), and in another five (6%), troponin rise without overt myocardial infarction was observed. The mean hospitalization time was 4 (3–5) days.
Discussion
Inflammatory cell counts may enable simple prediction of the location of calcific plaques in coronary arteries. Based on our analysis, lower peripheral neutrophil and lymphocyte counts are more representative of proximal, while higher counts are more representative for non-proximal calcific plaque location. These observations were independent of other, non-calcified lesions or clinical presentation on admission (stable or unstable angina). This is the first, to our best knowledge, study presenting the relation between the number of inflammatory cells and the location of calcified coronary lesions.
Previous reports suggested that the etiology of the proximal location of coronary artery disease may be explained by higher shear stress. Costopoulos et al. [15] described plaque structural stress determined by plaque composition and architecture and lumen geometry. The phenomenon was increased in plaques with rupture, particularly at proximal segments. The authors suggested the need for incorporation of the assessment of plaque structural stress into the coronary artery diagnostic protocol to improve the identification of rupture-prone plaques. Proximal lesions have also been related to smoking and dyslipidemia, and higher risk of unstable plaque, while distal lesions have been related to advanced age and diabetic factors [16]. In animal models, the proximal lesions related to the diet appeared more rapidly [17, 18]. Progression of coronary lesions has been associated with anatomic location and vessel diameter, with a higher frequency of proximally located lesions in large coronary arteries prone to rupture [19]. Moreover, the proximal and distal atherosclerotic lesions presented different histological compositions [9] due to diverse pathophysiological processes involved in the development and progression of the plaques. In Li et al.’s study [20] the differences between proatherogenic inflammatory and antiatherogenic inflammatory markers, including interleukins (IL) (IL-1β, IL-6, IL-8, IL-10, IL-17, IL-13, IL-37), TNF-α, and hs-CRP were related to different occlusion risk after percutaneous coronary procedures in the proximal, medial, or distal segments of the vessels.
Our analysis revealed the relation between neutrophil and lymphocyte count and the location of calcific atherosclerotic plaques. According to current knowledge and our previous reports in patients with ischemia treated with surgical revascularization [21, 22], the inflammatory background of coronary artery disease has already been accepted, though there are still several areas for scientific debate. Particularly, the understanding of individual patients’ differences in degree of inflammatory activation and its influence on long-term outcomes of the disease and interventional therapy is still lacking. Our previous reports [23, 24] have already proved that increased pre-procedural inflammatory activation, evaluated with simple indexes, may have good predictive value for the assessment of long-term survival or mortality. Thus, we suggest that some individual inflammatory characteristics may result in different responses to medical therapy. The current study concerns a particular stage of the disease with severe calcifications of coronary arteries and may help to explain several questions that may arise. The results showed that the increase of neutrophil and lymphocyte counts in peripheral blood analysis was predictive for non-proximal lesion location. Therefore, we believe that alterations in the inflammatory cell counts may be associated with different models of coronary artery progression. In previous studies, NLR was found predictive for the severity of coronary artery disease and clinical outcomes [22, 25]. So far, several analyses concerned the relation between the progression and severity of coronary disease and inflammatory activation presented as inflammatory indexes, including NLR, while lesion location has not been investigated [26, 27]. Therefore, we believe that further studies concerning cell phenotyping may enhance improvement in understanding the pathophysiology of coronary disease and explaining the different responses of individual patients to the implementation of similar therapies.
Patients enrolled in our study presented heavily calcified plaques where rotational atherectomy was necessary [28]. Balloon pre-dilatation of such plaques often leads to incomplete lesion dilation, increases the risk of uncontrolled vessel dissection and hampers optimal stent expansion, thereby worsening the prognosis [29]. High-pressure post-dilatation is not always effective and may lead to complications, such as extensive dissection or vessel perforation [30]. Rotational atherectomy has emerged as a potentially very effective technique for heavily calcified lesion preparation [31]. Recently, Farooq et al. [32] presented satisfactory results for RA in patients with heavily calcified lesions. Rola et al. [33] proved the safety and efficacy of RA in acute coronary syndromes.
The mobilized neutrophils initiate vascular damage and sustain an inflammatory environment by release of cytokines that play a significant role in atherosclerotic plaque [34]. Endothelial dysfunction is claimed to be responsible for atherosclerosis progression under the influence of cardiovascular risk factors [35]. The causative role of neutrophils is related to apoptosis induction, tissue factor production and breaking of the links between endothelial cells and the basement membrane [36]. Its significance in cardiovascular disease was proved in the CALIBER Cohort Study [37]. NLR was also presented as an atherosclerosis calcification predictor [38]. Naghedi et al. [39], in their review, highlighted the association between NLR and coronary calcifications. The relation between inflammatory markers, including NLR, and arterial stiffness in cardiovascular diseases, was described by Mozos et al. [40]. The aforementioned studies focused on atherosclerosis plaques’ characteristics, contrary to our analysis, in which we confirmed the importance of neutrophil and lymphocyte counts in predicting the location of atherosclerotic calcified coronary lesions. The novelty of our results is based on the parallel importance of both hematological indices in linear dependence. We believe that considering the lesion location, the interplay between neutrophils and lymphocytes may play a significant role. It must be underlined that neutrophils also exhibit beneficial effects during healing after myocardial ischemia and in tissue recovery [41].
Our results, by revealing the relationship between neutrophil and lymphocyte blood count and calcific lesion location, indicate the inflammatory background of coronary plaque formation and distribution. We believe that the results of our study will stimulate a new point of interest in the investigation of atherosclerosis progression. Further, larger studies are needed to clarify the role of white blood cells in coronary plaque formation, calcification and distribution.
Study limitation
The analysis was performed as a single-center, retrospective study based on a small number of patients referred for coronary rotational atherectomy for severely calcified lesions. Moreover, our study population includes patients with both acute and chronic coronary artery disease, though patients with acute myocardial infarction were excluded.
Conclusions
The number of inflammatory cells may differ depending on the location of significant calcified coronary lesions. The lower neutrophil and lymphocyte counts in peripheral blood count analysis may be more representative of proximal calcified coronary lesions. The relationship between neutrophil and lymphocyte blood count and calcific atherosclerotic plaque location may indicate the inflammatory background of epicardial atheromas formation and distribution.