Introduction
The progress in prompt and detailed breast diagnostics has enabled more reliable preoperative cancer staging and increased the variety of appropriate treatment options [1]. Among these options, nipple sparing mastectomy (NSM) with an immediate breast reconstruction provides oncological safety and a favorable cosmetic outcome in particular cases [2]. It does not adversely affect the timing of the adjuvant treatment. Moreover, it has become a preferable option in specific clinical settings, such as breast cancers characterized by multifocality or multicentricity. The term subcutaneous mastectomy is becoming increasingly common in studies regarding therapeutic options in the treatment of breast cancer [3-7], gynecomastia [8], the chest-wall contour change in transgender patients [9], risk-reducing prophylactic mastectomies, such as in BRCA gene mutation [10] and other rare indications [11, 12]. The presented paper analyses patients with breast cancers or BRCA mutation carriers. We aim to assess the postoperative findings in a cohort of patients undergoing this procedure, to evaluate complications and the correctness of the qualification of the patients for surgical treatment.
Material and methods
The study group consisted of 120 patients who underwent 130 NSM with a submuscular implant placement or tissue expanders between 1.01.2015 and 31.12.2017. The procedures took place in two hospitals in Poland: The Holycross Cancer Centre in Kielce (84 patients, 19 of them without cancer, 9 patients with bilateral mastectomy), and the Breast Cancer Unit in Mikołaj Kopernik Memorial Specialistic Center of Oncology and Traumatology in Lodz (36 patients, 21 of them without cancer, 1 patient with bilateral mastectomy).
The clinical and histopathological data were studied retrospectively. The group was divided into two subgroups: 80 patients diagnosed with cancer and 40 patients without cancer. The follow-up period was between 10 and 34 months (median 24 months). The surgical procedures were either therapeutic, for patients with cancer, or risk-reducing mastectomies (RRM), performed in BRCA1 mutation carriers. All patients underwent NSM. These were either performed as subcutaneous mastectomies, with conservation of the nipple-areola complex (NAC) or as skin-reduction mastectomies (SRM), with a free NAC transplant.
The patients were qualified for the aforementioned procedures when there was no cancer infiltration on the breast skin envelope, including NAC. In each case, separate histology specimens from the retro-areolar areas were examined with postoperative paraffin studies. No frozen intraoperative specimens were examined. When cancer infiltration was observed, the NAC was excised under local anesthesia in a post-operative out-patient clinic.
In 126 procedures, silicone microtextured implants placed under the pectoralis major muscle were used. Tissue expanders were used in four cases due to concerns regarding tissue pressure.
In the postoperative period, closed suction drains were used until drainage fell to less than 30 ml, or no longer than 10 days. One drain was used under the implant as a standard. In the case of axillary lymphadenectomy, an additional drain was provided. The length of antibiotic prophylaxis was continued until the removal of the drains. The antibiotic choice was concordant with local hospital policies.
The breast cancer patients received appropriate adjuvant treatment according to standard indications (chemotherapy, immunotherapy, hormonotherapy, radiotherapy).
Statistical analysis
The basic statistical measures (minimal, maximal, medium) for continuous features or frequencies and the percentage for original and quality features were used in the study. Mann-Whitney U test was used for features whose statistical division was not a common one for the evaluation of variables in two tested groups.
The Cox proportional hazard model was used to determine risk factors of complications. The following risk factors were included: preoperative and postoperative chemotherapy, hormonotherapy, radiotherapy, smoking, obesity, comorbidities. A p-value < 0.05 was considered statistically significant.
We have not asked for approval of the Ethics Committee due to the retrospective character of the analysis. Upon admission to hospital, the written consent of the patients to be used in the study was obtained.
Results
The cancer group consisted of 80 patients, with 84 mastectomies for oncological treatment (83 breast cancers and 1 case of malignant tumor phyllodes). The non-cancer group included 40 patients for other causes (risk-reducing mastectomies). The follow-up period was between 10 and 34 months (median 24 months).
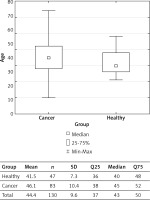
The mean age of all patients in both groups was 44.4 years (range 20 to 74).
The patients in the healthy (non-cancer) group were significantly younger (Mann-Whitney U test, p = 0.007) (Fig. 1). The majority of patients (113) were non-smokers, two were former smokers, and five were current smokers. Seven patients were obese (BMI > 30), while the other patients had a normal BMI. In 54 patients, BRCA1 mutation was confirmed pre-operatively. In four patients with breast cancer, different mutations were detected (CHEK2 in 2 patients, NOD in 1 patient). The most common comorbidity was arterial hypertension (Table 1).
Table 1
Comorbidities in all patients
Most patients in the cancer group were in stage T1 or T2. Multifocality or multicentricity was observed in 35 patients. In the cancer group, the mean distance between tumor and NAC was 43.3 mm (range 0 mm to 200 mm), while a larger distance (100-120 mm) was observed in 15 women. The diameter depends on the position of the skin incision. In 49 patients N0 status was staged. Two patients were diagnosed with distant metastases preoperatively. One patient was diagnosed with liver metastases but demonstrated a complete response to preoperative chemotherapy. Another patient was diagnosed with bone metastases; however, the disease had been stabilized with hormone therapy and the patient opted for NSM. The dominant tumor gradings were G2 (31 patients) and G3 (24 patients). The most common pathological finding was breast cancer of no special type (NST) (60 patients). Luminal types were prevalent. The clinical and histopathological data are given in Table 2.
Table 2
Characteristics of the cancer group
Surgery and adjuvant therapies
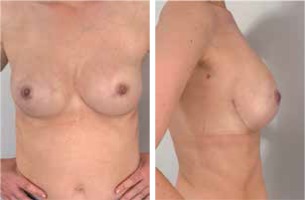
In cancer patients, adjuvant treatment was used (Table 3). Two patients were irradiated in the past following a breast-conserving therapy and currently operated due to recurrence. The mastectomies were bilateral in 10 patients (Fig. 2). In one patient they were performed during the same procedure, while for others, the mean period between the procedures was 133 days (range 21 to 293 days).
Oncological safety
The surgery and its complications did not delay the standard adjuvant therapy. No patient demonstrated local recurrence in our short follow-up. No histological report after RRM identified breast cancer. Despite no radiological evidence, post-operative histopathological examination revealed cancer nipple involvement in six patients. In these cases, NAC was removed in an out-patient clinic under local anesthesia.
Complications
We noted 14 cases of complications, 13 in the cancer group, and 1 in the non-cancer group. The mean time for the appearance of the complications was 2.8 months (range 0.1 month to 17 months). A capsule contracture was noted in three women who were treated with postoperative radiotherapy. In four cases, tissue expanders were used instead of implants to prevent excessive tissue pressure. Skin flap necrosis was recorded in two patients after skin-reduction mastectomy (one of whom received radiotherapy) and in three patients after NSM (two of whom received radiotherapy, and one received chemotherapy). Skin flap necrosis necessitated the removal of the implant in two out of five patients with skin necrosis, i.e. one diabetic and one obese patient. Skin necrosis was observed in one diabetic patient treated with an expander, necessitating its removal. Full-thickness necrosis of the NAC was observed in one woman after SRM and in three following NSM. In 1 smoker necrosis was observed. An implant dislocation was noted in two patients after NSM (Table 4). In the non-cancer group, superficial necrosis around the areola was noted. In a multivariate Cox regression analysis smoking was the factor which increased the risk of complications over twenty times (Table 5).
Table 4
Complications after the treatment including post-radiotherapy complications
Table 5
Cox regression analysis – complication risk factors
Discussion
Indications for subcutaneous mastectomy
In 2015-2017 in the Holycross Cancer Centre 773 mastectomies were performed, including 186 subcutaneous mastectomies with immediate reconstruction, which represented 24% of all mastectomies. The qualification for this procedure should be very prudent, because not every woman is a subject of this particular form of treatment. The style of life of the patient, comorbidities, age, body mass index, and forecasted oncological treatment can influence treatment outcomes.
Necrosis of the nipple-areola complex and skin
The combination of broad tissue undermining and a long skin wound can cause several complications. In the present study, nipple-areolar necrosis was observed. These are mentioned by other authors as the most common complications following a nipple-sparing technique [13, 14]. The safest area for surgical incision seemed to be the one performed in the lower quadrants of the breast [13]. Some new surgical strategies have been presented to avoid and resolve the necrosis of the NAC or skin [15].
Radiotherapy with nipple-sparing mastectomy
A considerable percentage of studied patients who presented ischemic changes of the skin envelope had previously undergone radiotherapy. Radiotherapy is mandatory for many patients with breast cancer; however, its side effects may be detrimental to the esthetic result of the NSM [16]. Interestingly, although an implant can reduce the radiotherapy dose, it does not appear to affect survival rates [17]. The optimal strategy is one based on a multidisciplinary approach at the stages of planning, treatment, and follow-up, with the patient included in the decision-making process.
Breast implant capsular fibrosis
Capsule formation around an implant is a well-known and extensively studied phenomenon [18]. Unfortunately, for the time being, there is no successful preventive measure. In our material, this complication necessitated another operation.
Nipple-sparing mastectomy after previous breast procedures
Previous interventions on the operated breast did not exclude the patient from NSM. It is regarded as a safe procedure [19].
Further surgery after nipple-sparing mastectomy
At times, more than one intervention is required for a favorable result [20]. Although some of the patients from our group underwent further procedures to improve the esthetic effect, these were performed outside our breast cancer units and the data are hence unavailable.
Oncological safety
Cancer relapses after subcutaneous mastectomies are rare [3, 4, 7, 21] as is the case in our present material. Such favorable outcomes may be attributed to a more detailed work-up of the patients for NSM and by the selection of less advanced cases, such as the exclusion of cases with skin involvement [2].
Malignant infiltration of the nipple-areola complex
During the present study, radiology studies and postoperative pathology reports were used to assess cancer infiltration of the NAC, as described previously [22]. Wong et al. report that the simultaneous excision of the nipple and an inferolateral incision could increase the incidence of skin flap necrosis [23]. In the present study, care was taken not to interrupt the delicate network of vessels in the 1 cm area around the areola. Postoperative paraffin histology examinations of the retro-areolar specimens were performed. In case of a positive finding, the NAC excisions were delayed until out-patient appointments. Other authors report the use of intraoperative frozen sections of the retro-areolar specimens for histology assessment and immediate NAC excisions in case of positive findings [5, 24]. Other studies suggest a classical counterindication for sparing the NAC complex; a two-centimeter distance between the tumor and the nipple, as indicated by the ultrasound scan and/or the magnetic resonance imaging, might not be necessary [25]. In case of a positive result for cancer histology in the retro-areolar shavings, excision of the nipple alone seems to give the best esthetic results. However, in those patients, we would recommend removing the whole NAC area.
Smoking and diabetes
Smoking and diabetes seem to be important risk factors for ischemic skin flap changes in the presented material and this view is shared in the literature [26]. Frey et al. even give precise cut-off points: the risk increases for > 10 pack-years smoking and/or < 5 YTQ (years-to-quitting) before NSM [27].
Breast size and obesity as risk factors
New independent risk factors for complications have been provided in the literature [28]. This is a mass of the postoperative specimen of > 700 g, distance from the inframammary fold to the nipple of >10 cm, and the width of the breast base of > 15 cm. It is possible that, in such cases, the surgeon could be more ready to use a larger implant or a tissue expander, and cause higher tissue tension. This could be the cause of the skin flap necrosis observed in one of the obese patients included in the study.
Standard adjuvant treatment with nipple-sparing mastectomy
At the beginning of the study, all the patients were informed about the potential complications associated with NSM procedures and were allowed to give informed consent. Standard guidelines for adjuvant therapy were applied during preoperative and postoperative scenarios for all patients with NSM. The proceedings were compliant with the data in the literature [29]. It has been reported that chemotherapy may show different effects in NSM, and hence there is a need for more precise clinical data [30].