Residual neuromuscular blockade (NMB) following general anaesthesia is associated with a prolonged length of stay (LOS) in the post-anaesthesia care unit (PACU) [1], unscheduled critical care admission [2], hospital readmissions [3], and postope-rative pulmonary complications [4, 5]. Routine monitoring of NMB reversal with train-of-four-ratio (TOFR) ensuring a value > 0.9 prior to extubation may reduce the incidence of postoperative pulmonary complications [6]. Despite evidence and professional guidelines mandating quantitative neuromuscular monitoring [7], clinical practice relies on clinical judgment and subjective assessments to determine the appropriateness of extubation [8]. This practice continues even though clinical tests such as head lift, grip strength, or spontaneous tidal volume are proven insufficient for ruling out residual paralysis [3–5].
To address the gap between standards and actual clinical practice, we undertook the Optimizing neuromuscular Blockade management for Improving patient Safety and Perioperative Outcomes (OBISPO) initiative. The primary goal was implementing clinical practice change emphasizing improved monitoring practices, ultimately reducing residual NMB. Our proposed two-phase quality improvement (QI) project focused on establishing a standardized NMB monitoring and pharmacological reversal strategy in the PACU and extending the change in care to the operating room (OR).
The initial phase of OBISPO, implemented in the PACU, primarily aimed to introduce a clinical practice change (clinician behaviour) by routine quantitative NMB monitoring. The secondary objective was to reduce residual NMB, defined as a TOFR of less than 0.9, in patients scheduled for fast-track extubation after elective cardiac surgery.
METHODS
This QI project included a cohort of all adult patients (aged ≥ 18 years) who underwent elective cardiac surgery and qualified for fast-track extubation at Toronto General Hospital PACU between February 1, 2021, and December 31, 2021. Approval for this study was obtained from the Institutional Research Ethics Board (UHN-REB), which operates in accordance with the Tri-Council Policy Statement of Canada and the International Conference on Harmonization-Good Clinical Practice Guidelines. The need for informed consent was waived by the REB given the QI nature of the project.
The primary outcome was assessed by evaluating the fidelity of implementation using run charts. The target was to record TOFR in at least 70% of the baseline cohort. Our secondary objective was to reduce residual NMB incidence before tracheal extubation to below 20% after the pilot phase.
An interdisciplinary QI team of staff anesthesiologists, fellows, residents, and PACU nurses was trained to implement and monitor practice changes. A series of Plan-Do-Study-Act (PDSA) cycles were conducted quarterly for root cause analysis, process mapping, evaluation of baseline data (1st quarter), development of improvement strategies (2nd quarter), education and feedback (3rd quarter), and overall assessment of practice change implementation (4th quarter).
Measures
Three types of measures were employed in this study:
Outcome measure: The primary outcome was the incidence of residual paralysis on PACU arrival, defined as a TOFR < 0.9. The secondary outcomes were PACU stay length, ventilation time, chest radiography request, reintubation, and desaturation events.
Process measure: TOFR recorded (Fidelity 70%).
Balance measure: NMB and reversal agents dosing during anaesthesia and in PACU.
All demographic preoperative, intraoperative, and postoperative variables were defined a priori.
Preoperative data included American Society of Anesthesiologists (ASA) classification (1, 2, 3, 4 or 5), surgery type, and estimated glomerular filtration rate (eGFR) calculated using the CKD-EPI creatinine equation.
eGFR values were classified as normal or elevated (G1: > 90), mildly decreased (G2: 60-89), mildly to moderately decreased (G3a: 45-59), moderately to severely decreased (G3b: 30-44), severely decreased (G4: 15-29) and kidney failure (G5: < 15).
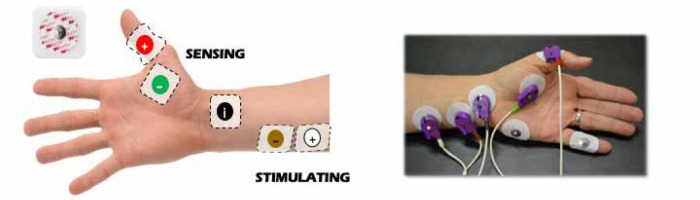
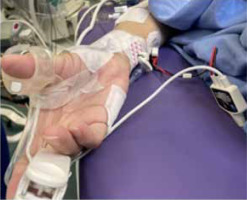
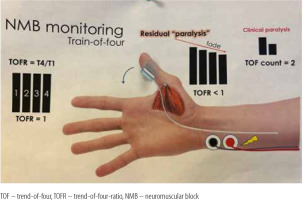
Datas were obtained from electronic records to build a single, de-identified database. TOFR was measured by either electromyography or acceleromyography using the following monitors: NMT Electron Sensor (GE HealthCare, Chicago, United States; Figure 1) and Philips Intellivue NMT monitor (Royal Philips Electronics, Amsterdam, The Netherlands; Figure 2).
PDSA Cycle 1: Root cause analysis, process mapping, and evaluation of baseline data
Initial meetings were held with the QI team to analyze the root cause using a fishbone diagram. The reasons for not monitoring NMB, such as (1) the absence of specific charts and alerts to monitor and document current practice, (2) practice traditions, (3) lack of awareness regarding current practice guidelines, (4) lack of reliable/sufficient monitors in the operating rooms and PACU areas, and (5) concerns about possible adverse events when using NMB monitors and pharmacological reversal agents, were discussed.
In addition to the absence of quantitative NMB recording, we observed a lack of protocol standardization for monitoring and administering pharmacological reversal agents resulting in variable and often unregistered clinical practices. When we performed process mapping, most elective fast-track cardiac surgery patients were extubated without quantitative NMB monitoring assessment, whereas pharmacological reversal was often not recorded.
PDSA Cycle 2: Development of improvement strategies
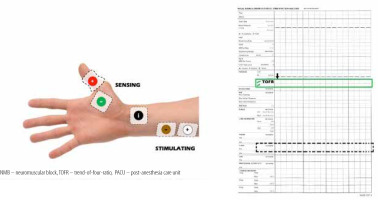
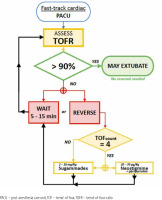
The QI team designed educational group sessions for personnel (residents, fellows, anaesthesia staff, and nurses) within the circle of care for the baseline patient cohort. Education focused on evidence supporting the current guidelines regarding the use of NMB monitoring, reversal, and operation of quantitative NMB devices available at our institution. In addition, cognitive aids were displayed in the appropriate care spaces (cardiac operating rooms and fast-track PACU bays), including a figure demonstrating electromyography electrode placement. A dedicated space for recording NMB assessments in PACU nursing charts, and electronic anaesthesia records were created (Figure 4) with an NMB management flowchart (Figure 5).
PDSA Cycle 3: Education and feedback
Educational refreshment training was held within the anaesthesia department by PACU nurses every two–three months, emphasizing the rationale behind NMB monitoring and reversal practice standards. During this cycle, new NMT devices (Intellivue NMT monitor) were purchased and placed at each anaesthesia station as an improvement strategy. This change required trained personnel to operate the new accelerometers (Figure 6).
PDSA Cycle 4: Implementation assessment
After the results from the previous cycle, educational rounds were reinforced; we displayed the trends of ongoing outcomes. To understand the weaknesses and gaps in knowledge to be addressed at the end of the first year, all staff, residents, and fellows completed a survey (Table 4) regarding the use of NMB monitoring and the factors associated with the lack of use of quantitative NMB monitoring as the standard of care.
Statistical analysis
SPSS Statistics (IBM, USA, release 28.0) was used to analyze the data. The baseline characteristics of the patients, TOFR, residual paralysis, use of NMB reversal agents, complications, time of ventilation, and LOS in the PACU were calculated and presented as percentages (numbers), medians (percentiles), or means (standard deviations).
RESULTS
Of the 859 patients who underwent elective cardiac surgery at our institution between February and December 2021, 341 (40%) were transferred to the PACU for fast-track extubation. The population consisted of 238 (70%) male subjects with a median age of 62 years old (IQR [54–70]). Of the 341, 64 (19%) were classified as ASA 3 and 277 (81%) as ASA 4. Most of the patients had normal (152 [94%]) or mildly decreased (157 [46%]) eGFR.
Demographics, including the type of surgery, are presented in Table 1.
TABLE 1
Overall results, patient’s characteristics, and type of surgery
In the baseline cohort, on arrival at PACU, 236 (69%) TOFR were registered; 111 (47%) had TOFR < 0.9 before extubation, 24 (22%) had residual paralysis after extubation, 91 (27%) were extubated without TOFR monitoring, and 33 (10%) were transferred intubated to intensive care unit (ICU). All the patients received rocuronium as a paralyzing agent; the mean dose was 1.12 mg kg–1 (0.23 mg kg–1 h–1). NMB reversal agents were used in 85 (25%) patients, and neostigmine in 61 (72%). During surgery, 146 (43%) patients received NMB reversal agents, 140 (96%) received neostigmine without quantitative NMB monitoring or confirmation of TOFR > 0.9 before arrival to PACU, 55 (39%) patients received < 0.04 mg/kg as a single dose (Table 2).
TABLE 2
Register of neuromuscular blockade in PACU, doses of neuromuscular reversal agents and doses of rocuronium in OR
The overall results showed that patients had a mean ± standard deviation (SD) LOS in the PACU of 87 ± 56 minutes (min) with a ventilation time of 55 ± 14 min. During their stay in PACU, eight (2%) patients underwent chest radiography, thirteen (4%) patients required increased oxygen delivery after extubation with no desaturation (< SpO2 90%) events observed, and no patients required reintubation (Table 2).
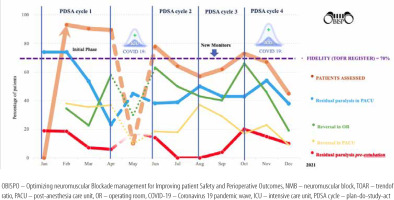
Over 46 weeks, and four PDSA cycles, the evolution of results included the percentage of patients eligible for fast-track extubation after elective cardiac surgery, TOFR register (fidelity) in the PACU, residual paralysis on arrival at the PACU, residual paralysis pre-extubation, and use of reversal in the OR and PACU (Figure 3). The effects of each PDSA cycle are presented in Table 3.
TABLE 3
Results of PDSA cycles
During PDSA cycle 1 (February to April 2021), 79 (40.5%) patients were eligible for fast-track. The mean TOFR registered was 91%, 39 (54%) patients had residual paralysis on arrival at PACU, 28 (35.4%) patients received NMB reversal in OR, and 29 (36.7%) of them in PACU. No reintubation was observed, ten (12.6%) patients were extubated in ICU, three (3.7%) patients required an increase in oxygen delivery, and one (1.2%) patient had chest radiography. The mean ventilation time was 72 ± 7 min, and the LOS in the PACU was 120 ± 21 min.
From May to June 2021, the COVID-19 pandemic’s second wave interfered with the study because all elective cardiac procedures were cancelled. During this period, training was provided to PACU nurses, and evaluation of the study progress was conducted.
During PDSA cycle 2 (June to July 2021), 55 (35.9%) patients were eligible for fast-track extubation. The TOFR register decreased, but the number of patients with residual paralysis also decreased. Fewer patients were extubated in ICU (12.6% vs. 5.4%) and no adverse events were observed. The use of NMB reversal in the OR increased, with a decrease in the use of reversal agents in the PACU. The duration of mechanical ventilation decreased but the LOS in the PACU increased.
New NMT devices were obtained between cycles 2 and 3. During the PDSA cycle 3 (August to September 2021), 80 (43.7%) patients were eligible for fast-track extubation, the TOFR register decreased by 10%, and residual paralysis persisted in 27% of the patients. A decrease in NMB reversal in the OR was observed (56.3% vs. 41.2%), with reversal agents use in PACU increasing from 18.1% to 32.5%. Regarding complications, five (6.2%) patients required an increase in oxygen delivery, two (2.5%) patients required chest radiography, and no reintubation episodes were observed. The ventilation time decreased by 40 ± 24 min, and the LOS in the PACU decreased to 88 ± 24 min compared to those in cycle 2.
The survey performed (Table 4) during PDSA cycle 4 (October to December 2021) consisted of three questions. Overall, we observed that 29 (56.8%) respondents used clinical assessment to assess NMB recovery and had difficulties using quantitative NMT monitors due to a lack of knowledge or bias, even though 23 (50%) of the responders were staff with more than five years of experience.
TABLE 4
Survey: Use of NMB monitorization and factors associated with the lack of use of quantitative NMB monitoring as the standard of care
During the 4th PDSA cycle, the third wave of COVID-19 impacted clinical care. Of 275 patients, 127 (46%) were eligible for fast-track cardiac extubation. The TOFR register decreased (60.6%), with a further increase in residual paralysis to 43% and an increase in the number of patients extubated in the ICU as well (11%). However, the use of NMB reversal agents in the OR increased to over two-thirds, with a commensurate decrease in the PACU. An increase in oxygen delivery was required in five (6.2%) patients and chest radiographs in five (6.2%) patients. An increase in ventilation time and LOS in the PACU was observed compared to that in cycle 3 (Table 3).
Discussion
The main results of our study showed that a significant proportion (47%) of patients qualifying for fast-track extubation after elective cardiac surgery had residual paralysis upon arrival in PACU. Furthermore, 22% had residual paralysis after extubation, and 27% were not monitored using NMT. At the end of the initial phase, residual paralysis remained at 38%, but increased use of reversal agents was observed in the last PDSA cycle (67.5%).
These measurements improved over the study period, reaching a minimum fidelity of 70% for TOFR monitoring in the overall results. Historically, NMB assessment at our institution has been limited, primarily subjective, and poorly documented. Through our initiative, we addressed some of the root causes and changed the behaviour of the perioperative team responsible for monitoring and managing NMB.
Additionally, after the completion of the first part of this QI study, quantitative NMT monitoring became part of clinical practice in the PACU for fast-track cardiac surgical patients in our hospital. TOFR was routinely recorded in patients’ electronic charts, and reversal agents were administered according to our protocol.
The results of our project complement the existing literature. Recently published guidelines by the American Society of Anesthesiologists (January 2023) recommend the routine use of quantitative TOFR monitoring [9], which corresponds to our project. All other major anaesthesia professional organizations, including the Canadian Anesthesiologists’ Society [7], the European Society of Anesthesia and Intensive Care [10], and the Association of Anesthetists of Great Britain and Ireland [11], have strongly advised the use of quantitative NMT monitoring for many years. Moreover, evidence-based recommendations are based on multiple studies showing that residual muscular paralysis is a major contributor to postoperative respiratory complications [5].
Several initiatives addressed adherence to routine quantitative NMB monitoring in patients undergoing surgery with the use of NMB monitors. A recent publication by Díaz-Cambronero et al. [12] described an effort to reduce residual paralysis in the postoperative period using an educational intervention across 34 centres in Spain. This study demonstrated that clinical practice behaviour changes were challenging; during their study, an educational aid regarding the use of quantitative NMT and reversal of NMB was used in multiple centres for six months. After this period, the investigators concluded that the educational intervention did not reduce the incidence of residual paralysis. However, they noticed an increase in the use of NMT and sugammadex as NMB reversal, with an associated decrease in postoperative pulmonary complications.
Weigel et al. [6] conducted a similar QI project in a single institution to monitor all patients in the OR to prevent residual NMB before tracheal extubation. They initially faced resistance from care providers regarding the adoption and use of quantitative NMB monitoring. After the full implementation of their QI initiative, TOFR > 0.9 improved from 1% to 92%. Attaining this endpoint required quantitative monitor placement in each anaesthetizing location and ongoing four-year educational efforts to elicit behaviour change. Both general and individual performance feedback systems were used. Additionally, real-time automated anaesthesia charting reminders and departmental leadership requesting TOFR documentation as an ongoing professional practice evaluation metric were fundamental to achieving success.
Rudolph et al. [13] evaluated the effects of a QI initiative optimizing neostigmine use to antagonize NMB and its effect on postoperative pulmonary complications, costs, and duration of hospital stay. The authors demonstrated that altered practice behaviours included neostigmine dose reductions, decreased excessive neostigmine doses, and improved intraoperative TOFR documentation. Furthermore, the QI initiative was associated with reduced hospital stay and costs.
Bedsworth et al. [14] developed an initiative to improve anaesthesia providers’ knowledge of NMB pharmacology, physiology, and monitoring. After completing their project, an analysis was conducted to assess changes in practice regarding the use of quantitative monitoring and dosing of NMB agents and neostigmine. Quantitative monitoring increased significantly from 14.0% in the pre-initiative group to 48.0% after the completion of the QI project. This quantitative monitoring initiative successfully increased the use of quantitative NMB monitors; however, it did not affect providers’ overall dosing practices for NMB agents or neostigmine. This study stated that adopting research findings into routine clinical practice is a slow process that sometimes fails to sustain long-term practice changes, as evidenced by a decrease in NMT monitoring over time.
Drzymalski et al. [15] conducted a single-center study that included cognitive aid to help clinicians to choose between NMB reversal agents. This project was developed due to an increased cost after the introduction of sugammadex in their institution. The authors hypothesized that sugammadex use and the associated costs would decrease after the introduction of educational aids. This initiative compared sugammadex administrations and costs before and after aid application. To perform an interrupted time-series analysis, an investigation of the baseline situation regarding the number of sugammadex vials was used. The associated cost was analyzed before initiative initiation, and monthly progress evaluation was conducted. After 21 months, there were 509 fewer sugammadex administrations than predicted per 1,000 general anaesthetics. This study facilitated significant change in clinical practice but took two years of education to change clinical behaviour. In the discussion, the authors acknowledge that the outcome was not only related to the aid itself but multiple factors, especially collaboration from multiple departmental leaders and continuous reinforcement of its importance were involved.
In summary, all the aforementioned studies showed that QI projects aimed at improving quantitative NMB monitoring and safe extubation (TOFR > 0.9) required multiple interventions and required several years to achieve their goals.
Our study had several limitations. The COVID-19 outbreaks affected our study, with cancellation or postponement of elective surgeries. Consequently, the training and knowledge were outside of routine practice once the PACU was transformed into a regular ICU. Later, during the study, a different brand of quantitative NMB monitor replaced those used at the beginning of the study (both accelerometers, but with different limitations). This change affected the results of the next evaluation cycle, and new training and adherence to the changes in devices were required. We observed that, regardless of persistent training and education, a high incidence of residual paralysis (27.5%) was observed at the end of the study. This result is potentially related to multiple changes in NMT monitors and the short study time, implying that more time is required to change clinicians’ behaviour.
Finally, changes in clinical practice are challenging, and continuing education with process evaluation is mandatory. The time required to observe a significant difference in behaviour represents the prolonged period required for educational interventions to merge with physicians’ practice. In this context, our observation period was relatively short; however, we focused only on the first part of the OBISPO initiative.
CONCLUSIONS
Our study showed that clinician behaviour change is one of the most challenging issues in the perioperative care of patients requiring NMB. Despite these challenges, we were able to achieve several goals, and TOFR is now routinely monitored before extubation following cardiac surgical procedures. Continuous teaching and QI initiatives that focus on the use of quantitative NMB monitors and adequate administration of reversal agents are mandatory to progress and ultimately improve perioperative outcomes, including the reduction of postoperative pulmonary complications. Changing current practices regarding the safe use of NMB agents remains difficult. Therefore, new proposals and studies are required to promote changes in current practice and clinicians’ behaviour.