Introduction
Androgen deprivation therapy (ADT) was the standard treatment of hormone-sensitive metastatic prostate cancer until recently. Despite the high response of patients with prostate cancer to ADT, the majority of them progressed to castration-resistant prostate cancer (CRPC) within 1–3 years [1]. Hormone-sensitive prostate cancer cells may become resistant, because some cells may have been hormone resistant from the start [2]. Autocrine androgen production, amplification of androgen receptor (AR) protein, and other mechanisms that bypass the AR such as coactivators and trans activators are theories to explain the resistance. Some of the most important of these biologically heterogeneous mechanisms involve cancer stem cells, receptor tyrosine kinases, and neuroendocrine differentiation [2].
Three large randomized trials, STAMPEDE (arms C and E) [3], GETUG-AFU 15 [4], and CHAARTED [5], showed the benefits of adding docetaxel to standard ADT as regards to biochemical progression-free survival, clinical progression-free survival and overall survival (OS).
Patient nutritional status and immunity play an important role in cancer metastasis and progression [6]. Recent studies showed that neutrophils, lymphocytes, platelets, and monocytes play an important role in tumor invasion and metastasis [7].
Growing data confirm that the tumor microenvironment, which is largely coordinated by inflammatory cells, is an indispensable participant in the neoplastic process, fostering proliferation, survival, and migration. In addition, tumor cells have co-opted some of the signaling molecules of the innate immune system such as selectins and chemokines with their receptors for invasion, migration, and metastasis. These insights are fostering new anti-inflammatory therapeutic approaches to cancer development [8].
Based on these data, the inflammatory index called the HALP score, which is composed of the calculations from hemoglobin, albumin, lymphocytes, and platelets, is proved to be a prognostic index in bladder, colorectal, and renal cancer [9].
In this study, we aim to confirm the benefits of treatment with docetaxel and ADT as regards to both efficacy and tolerability in newly diagnosed high-tumor-burden hormone-sensitive prostate cancer (mHSPC) patients. Moreover, we tried to evaluate usage of the easy, bedside, and non-invasive HALP score as a prognostic model which helps to stratify patients and predict the benefit from therapy.
Material and methods
This prospective study was performed at the Medical Oncology Department, Clinical Oncology and Nuclear Medicine Department, Pathology Department, and Urology Department, Faculty of Medicine, Zagazig University, Egypt from March 2019 to March 2021. It was approved by the Ethical Committee (Ethical code: 6991); written informed consent was taken from all included patients. We included a total of 50 naïve patients with high-burden mHSPC. High-burden disease is defined as either more than or equal to 4 bone metastases, with at least one outside the pelvis, vertebral column, or visceral areas [1].
Transrectal ultrasound guided biopsy was done at the Urology Department. Specimens were processed and examined for histopathology at the Pathology Department. Staging workup was evaluated by whole-body bone scintigraphy and computed tomography or magnetic resonance imaging (MRI) scans. Baseline laboratory data including complete blood count with differential, liver function tests, and renal function tests were obtained before the treatment. Prostate specific antigen (PSA) was measured for all patients as a baseline test then repeated at the following intervals: every other cycle of chemotherapy, every 3 months during chemotherapy and during the first 2 years of follow-up, and subsequently every 6 months.
Patients received ADT+ docetaxel (75 mg/m2) with prednisolone 5 mg twice daily for six cycles (once every 3 weeks). Additionally, patients received ADT in the form of a luteinizing hormone-releasing hormone agonist plus anti-androgen (bicalutamide), or surgical orchidectomy plus anti-androgen. Treatment was discontinued if there was progression of the disease or toxicity from medication. Chemotherapy delay for one week was seen in 4 patients due to neutropenia; subsequent doses of prophylactic growth factor rescued them in subsequent cycles without need for dose reduction. The primary end-point of the study was the efficacy of treatment and tolerability. The secondary end-point was the clinical value of the HALP score in predicting response to treatment and survival outcome. HALP score was calculated as hemoglobin level (g/l) × albumin level (g/l) × lymphocyte (/l)/platelet count (/l) [10].
Patients’ exclusion criteria
Patients with low metastatic burden disease from the start, patients with small cell variant or patients who had more than one type of cancers.
Prostate specific antigen complete response was defined as a decrease to 0.2 ng/ml, to be confirmed by a second PSA measured 4 weeks later. Increase in the PSA level by 50% above the nadir was confirmed by another assessment after at least 2 weeks, which indicates disease progression. For patients with a PSA nadir of 2 ng/ml, a PSA value of ≥ 2 ng/ml was required to confirm disease progression by PSA only and qualified as CRPC [6]. Computed tomography scans of the chest, abdomen, and pelvis, bone scan, and MRI were done according to physician decision and biochemical failure, or when the patient was symptomatizing. Response Evaluation Criteria in Solid Tumors (version 1.0) was the scale used for disease evaluation with measurable lesions [11].
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 5.0) [12].
Statistical analysis
Quantitative data were expressed as the mean ±SD and median (range) while qualitative data were expressed as an absolute frequency (number) and relative frequency (percentage). Continuous variables were checked for normality using the Shapiro-Wilk test. The Mann- Whitney U test was used to compare between two groups of non-normally distributed variables. The Kruskal-Wallis H test was used to compare between more than two groups of non-normally distributed variables. Percentages of categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test when it was appropriate. Time to CRPC was calculated as the time from the date of starting the chemo-hormonal treatment to the date of proving CRPC, or the most recent follow-up in which the patient was free from castration-resistant disease. Overall survival was calculated as the time from diagnosis to death or the most recent follow-up contact (censored). Stratification of time to CRPC and OS was done according to HALP score. These time-to-event distributions were estimated using the method of the Kaplan-Meier plot, and compared using the two-sided exact log-rank test. All tests were two sided. A p-value < 0.05 was considered significant. All statistics were performed using SPSS 22.0 for Windows (IBM Corp., Armonk, NY, USA) and MedCalc 13 for Windows (MedCalc Software bvba, Ostend, Belgium).
Results
Clinicopathological characteristics
The median age of the fifty patients of our study was 69.5 years old (range: 60–79 years old). Twenty-one patients (42%) were obese (BMI ≥ 30). Thirty-two patients had a Gleason score from 8 to 10. All study patients had Eastern Cooperative Oncology Group performance status 0–1. Fourteen patients had bone metastasis only while seven patients had lung metastasis, and eleven patients had liver metastasis. Mean pre-treatment PSA was 202.57 ng/ml with median value ranging from 5.35 to 780 ng/ml while mean post-chemotherapy treatment PSA was 32.67 with median value ranging from 0.03 to 220 (Table 1). The mean value of the pretreatment HALP score was 25.78 with the median value ranging from 7.50 to 68.80.
Table 1
Clinicopathological characteristics of hormone-sensitive prostatic carcinoma patients
Efficacy of therapy
In our study a CR (complete response) was achieved in 9 patients (18%) while PR was seen in thirteen study subjects; therefore, the overall response rate (ORR) was 44%. The median time to CRPC was 12 months for the whole study population; however, time to CRPC was more than 12 months in 24 patients (48%) and was ≤ 12 months in 26 patients. At time of this analysis, nine of our patients have died while 41 patients are still alive; mean OS was 21.26 month and the 2-year OS was 80.2% (Table 2).
Table 2
HALP score stratified by toxicity and outcome among hormone-sensitive prostatic carcinoma patients (N = 50)
HALP score stratified by outcome
Regarding all parameters of benefit from our treatment (chemo-hormonal therapy) there was a significantly higher HALP score in those with a complete response (p-value = 0.001), those with an overall objective response (CR + PR) (p-value < 0.001), those with time to CRPC more than 12 months (p-value < 0.001) and also in alive patients compared to dead patients (p-value = 0.034) (Table 2).
Relation between HALP score and response to treatment
There was a significant association between HALP score and response to treatment. In fact, a higher rate of complete response occurred in patients with a high HALP score than patients with a lower HALP score (53.8% vs. 5.4% respectively, p-value = 0.001); also, a significantly higher rate of overall objective response (CR + PR) occurred in patients with a higher HALP score than patients with a lower HALP score (76.9% vs. 32.4% respectively, p-value = 0.005) (Table 3).
Table 3
Relation between HALP score and toxicity/outcome among hormone-sensitive prostatic carcinoma patients
Relation between HALP score and time to castration-resistant prostate cancer
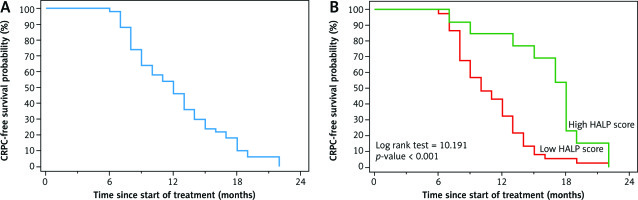
Patients with time to CRPC more than 12 months had a significantly higher HALP score compared to patients with a lower HALP score (84.6% vs. 35.1% respectively, p-value = 0.002); also, there was a significantly higher median time to CRPC in patients with a high HALP score than those with a low HALP score (median was 18 and 10 months, respectively). Eighteen-month CRPC-free survival was significantly higher in patients with a high HALP score than patients with a low HALP score (23.1% and 5.4% respectively, p-value < 0.001) (Fig. 1, Table 3).
Association between HALP score, mortality and overall survival
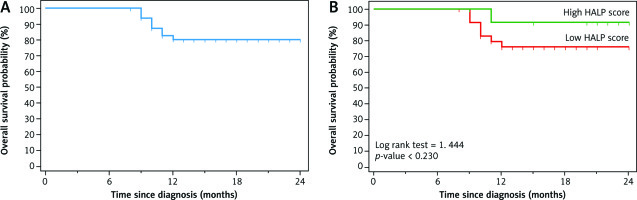
There was no significant association between HALP score and mortality (p-value = 0.414). Patients with a high HALP score had insignificantly higher mean OS than patients with a low HALP score (mean OS was 22.91 and 20.66 months respectively). Moreover, 24-month OS was insignificantly higher in patients with a high HALP score than patients with a low HALP score (91.7% and 76.1% respectively, p-value = 0.230) (Fig. 2, Table 3).
HALP score stratified by toxicity
Forty-one patients (82%) developed manageable toxicity from treatment; most of them were grade I. The most common adverse events were anemia (46%) followed by neurotoxicity and fatigue (32%) (Table 4). There was an insignificant difference between patients who developed toxicity and those who did not develop it, regarding the HALP score (p-value = 0.733). In contrast to patients who did not have neutropenia, they had a significantly lower HALP score in comparison to those who developed it (mean ±SD was 22.60 ±15.76 and 38.50 ±19.67 respectively, p-value = 0.013) (Table 2). A significantly higher rate of neutropenia occurred in patients with a high HALP score than patients with a low HALP score (46.2% vs. 10.8% respectively, p-value = 0.012) (Table 3).
Table 4
Toxicity of chemo-hormonal treatment among hormone-sensitive prostatic carcinoma patients
| Toxicity of chemo-hormonal treatment | All studied patients (N = 50) | |||
|---|---|---|---|---|
| G1 | G2 | G3 | Percent | |
| Any toxicity | 41 | 82 | ||
| Neurotoxicity | 10 | 6 | 0 | 32 |
| Neutropenia | 5 | 5 | 0 | 20 |
| Thrombocytopenia | 6 | 0 | 0 | 12 |
| Anemia | 13 | 6 | 4 | 46 |
| Fatigue | 12 | 4 | 0 | 32 |
All the remaining parameters of toxicity including thrombocytopenia, anemia, and fatigue showed no significant difference between patients who did not develop vs. those who developed toxicity regarding HALP score (p-value = 0.324, 0.675, and 0.441 respectively) (Table 2).
Relation between HALP score and toxicity
No significant association was found between toxicities and HALP score except for neutropenia, where higher rates of neutropenia significantly occurred in patients with a high HALP score than patients with a low HALP score (46.2% vs. 10.8% respectively, p-value = 0.012) (Table 3).
Discussion
Adding chemotherapy to hormonal therapy in treatment of naïve high-tumor-burden mHSPC improves the outcome, and has become the standard of care. Long-term analysis of studies confirmed the clinical benefit regarding better survival and time to disease progression compared to ADT alone [13].
In this study – when docetaxel was given in combination with ADT for high-burden mHSPC – better disease control was obtained, time to develop CRPC reached < 12 months in 48% of patients, and the ORR was 44%. This was in line with the study of Christopher et al., who reported better control of cancer with longer time to develop CRPC, and longer OS with a median OS of 49.2 months. However, in our study the mean OS reached only 21 months, but this difference may be due to the difference in the follow-up period, which was longer in the study of Christopher et al. [1].
In the CHAARTED trial, the median OS in patients who were treated with docetaxel plus ADT in the high-burden group was 51.2 months, with median time to CRPC of 14.9 months [5]. This did not match the results of our study with mean OS = 21 months (median not reached), and median time to CRPC was 12 months. However, this difference between the studies seemed to be due to the short follow-up period in our study and the smaller number of patients.
The most common adverse events in this study were anemia (46%) followed by neurotoxicity and fatigue (32%); nevertheless, these did not interfere with patients’ quality of life (QOL) and were manageable with no chemotherapy discontinuation due to adverse effects. Also, in the CHAARTED trial, docetaxel was associated with decreased QOL on treatment (at 3 months) which was not noted in treatment with ADT alone. However, 12-month QOL was better for the patients who received docetaxel vs. ADT alone. For this reason, docetaxel + ADT did not appear to have a long-term negative impact on QOL for mHSPC, which agreed with our results [14].
Furthermore, we tried an easily counted predictive index, i.e. the HALP score, to predict disease outcome in high-burden mHSPC patients who received docetaxel plus ADT. Additionally, the calculation of the HALP score – as a new prognostic index to evaluate the impact of response to treatment, and the progression to CRPC – was observed to be an independent risk factor for those patients.
Recently, nutrition and immunity have gained attention as prognostic factors in cancer patients [15, 16]. Furthermore, hemoglobin level has been noted to be highly related to tumor progression and survival [17]. The HALP score, which was calculated in our study as hemoglobin level (g/l) × albumin level (g/l) × lymphocyte (/l)/platelet count (/l), with a mean score of 25.78, indicated that patients with a low HALP score where hemoglobin is one factor of this score experienced tumor progression and a poor response to therapy; this was compatible with the study of Caro et al., who identified anemia as an independent prognostic factor for poor prognosis with increased risk of death (47%) [18]. The low HALP score in our study was significantly higher in dead than alive patients, where mean ±SD was 17.86 ±16.02 and 27.52 ±17.63 respectively (p-value = 0.034)
Also, serum albumin reflects the level of protein to assess the nutritional status of patients; this is supported by the literature to be correlated with survival [19]. Sejima et al. supported the hypothesis that preoperative low albumin level enhances early biochemical failure and rapid spread of localized prostate cancer [20]. Lymphocytes have an obvious function in suppression of tumor proliferation, invasion, or metastasis. So, lymphopenia occurs more frequently in advanced cancer [9].
Furthermore, lymphopenia is an independent prognostic factor for poor prognosis in cancer patients [21]. Some studies have demonstrated the relation between platelets and tumor microenvironment which was revealed by different mechanisms that increase tumor progression and metastasis [8]. So, the combination of these factors can be used as novel index, the HALP score, in various studies; this was inferred from the fact that low HALP score was an independent poor prognostic factor for disease progression and poor response to therapy, which was confirmed by our study.
HALP score was first described by Chen et al. as a prognostic factor in gastric cancer [22]. Subsequently, it was used in predicting the prognosis of renal cell carcinoma [9], bladder cancer, prostate cancer, colorectal cancer, esophageal cancer, pancreatic cancer, and lung cancer [23–25].
Guo et al. observed the relation between HALP score and PSA-progression free survival in patients with metastatic prostate cancer after radical prostatectomy; it was an independent prognostic factor for poor prognosis. Also, they mentioned the association between low HALP score, tumor progression, and poor outcome [26]. Compared to our study, it was observed that low HALP score had a significant relation with poor prognosis, tumor progression, and short time to CRPC less than 12 months; on the other hand, patients with high HALP score had a higher rate of complete response than patients with a low HALP score (53.8% vs. 5.4% respectively, p-value = 0.001).
In our analysis, patients who had a low HALP score at diagnosis had a poor outcome and less than 12-month time to develop CRPC; meanwhile, patients with a high HALP score had a better prognosis with more than 12-month time to develop CRPC.
Therefore, in the context of these data, HALP score is a new predictive and prognostic score – correlated with cancer patients’ prognosis, response to treatment, and survival – with an obviously better response in those with higher hemoglobin, albumin, and lymphocytes and a poorer response for those with higher platelet counts.
So, we inferred that there is high predictive value of the HALP score to classify patients into high and low risk of recurrence and tumor progression, to provide more suitable treatment options according to risk groups.
Limitations of the study
The following are some limitations of our study: first of all, the sample of patients was small, and they all received the same line of treatment with no comparative groups. The second is the short follow-up period; more accurate results could be achieved if the follow-up period was prolonged. Further studies with a larger sample size and longer follow-up period are recommended.
Conclusions
The combination of standard ADT and six cycles of docetaxel were well tolerated, with significant clinical benefit in patients with high burden disease. Furthermore, the availability of a new prognostic HALP score which is a low cost, fast and accurate marker appears to improve the accuracy of prognosis of metastatic prostate cancer and can help to make a precise decision to choose the proper treatment. In our analysis patients who had a low HALP score at diagnosis had a poor outcome and a short time (less than 12 months) to develop CRPC. In contrast, patients with a high HALP score had a better prognosis with the time to develop CRPC being more than 12 months. Thus, patients should be stratified not only according to burden of the disease, but also according to this score into high and low risk groups, to allow better choice of appropriate treatment according to risk stratification.