Ovarian reserve evaluation after laparoscopic cyst enucleation, depending on applied haemostasis technique and with particular consideration of endometrial cysts.
Endometriosis is defined as an extrauterine occurrence of adenocytes (gland cells) and of endometrial stroma. The percentage of diagnosed cases of endometriosis varies – acc. to various estimates – from 6 to 10% of women in reproductive age [1]. Of the numerous theories that explain how the onset of endometriosis develops, a few can be isolated and grouped into the following three categories: theory of endometriosis development in situ, theories of transmittable infections, and inductive theories. Among the theories of transmittable infections, the theory of retrograde menstruation, proposed by Sampson, should be perceived as leading. It is based on the observation of a backward flow of some menstrual blood through the ovarian ducts to the peritoneal cavity. This flow carries live and able for implantation, desquamated cells of the endometrium [2]. This theory does not, however, explain all the doubts which are associated with the pathogenesis of the disease. If retrograde menstruation occurs in 90% of women, then why does endometriosis develop only in some of them? The answer to this question has, in part, been provided by the theory of immunological deficit [3]. According to this theory, disturbed functions of macrophages, NK cells, and cytotoxic lymphocytes of the peritoneal fluid, observed in women with endometriosis, are significant for survival of displaced endometrial cells. These cells are responsible for phagocytosis of erythrocytes, morphotic elements of menstrual blood, or damaged tissue fragments. A number of other disturbed responses of the immune system, both cellular and humoural in character, were found in the peritoneal fluid of women with endometriosis [4].
The disease is manifested mainly by pain sensations, compromised fertility, menorrhoea disturbances, or by a concomitant incidence of these disorders [5]. The ovary is a frequent location of endometriosis – perhaps for its surface irregularities, which favour the formation of endometrial cysts [6]. A standard procedure in the surgical treatment of endometrial cysts is pseudocapsule enucleation [7]. The stripping method – as it were – involves the use of two atraumatic gripping instruments to pull away the cystic capsule and the normal ovarian parenchyma in opposite directions.
A systematic review and meta-analysis indicate, however, a negative effect of ovarian cysts capsule enucleation on the ovarian reserve. The commonly acceptable markers of ovarian reserve include the anti-Mullerian hormone (AMH), the antral follicle count (AFC), the follicle-stimulating hormone (FSH), and inhibin B. There is a problem with normal antral follicles, which may be either removed in the course of capsule enucleation procedure or damaged during haemostasis, achieved by means of electrical energy or intraperitoneal suturing. On one hand, the use of electrical energy requires very high precision and accuracy due to the risk of damage to the surrounding normal ovarian tissue, while intraovarian suturing may increase the intraovarian blood pressure, leading to local ischaemia on the other. Therefore, the selection of an appropriate technique of haemostasis still remains a rather controversial issue, thus demanding further research, comparing ovarian coagulation with ovarian suturing in female patients undergoing the surgical procedure of ovarian cyst enucleation.
Aim of the study
The goal of the reported study was an evaluation of the effects exerted by applied haemostasis techniques on ovarian reserve following laparoscopic enucleation of endometrial cysts.
Material and methods
The study was carried out during the years 2014-2016 in patients admitted to the Department of Endoscopic Surgical Gynaecology and Oncological Gynaecology. Female patients, at the age of 20-35 years, were included into the study with cystic lesions of 40-70 mm in diameter. All the patients provided written, informed consent to participate in the clinical study on a specially prepared form approved by the Bioethical Committee.
The patients were randomly assigned to two study groups. Group 1 involved patients after laparoscopic enucleation of ovarian cysts, in whom haemostasis was achieved by ovarian suturing, while group 2 included patients with haemostasis achieved by bipolar coagulation technique. Patients with history of cyst enucleation were disqualified from the study. In total, 66 patients were qualified and subsequently submitted to cyst enucleation procedure. The mean age of the patients in either group was similar at 31 ±5.18 vs. 33 ±4.78 years (p = 0.871).
In 33 patients, haemostasis was achieved by suturing of the ovary. The sutured group included 24 cases of diagnosed endometrial cysts, six cases with dermoid cysts, and three with simple cysts. In group 2, haemostasis was done by electric energy. The coagulated group included 16 cases of diagnosed endometrial cysts, eight cases with dermoid cysts, and nine with simple cysts.
Preoperative blood samples provided material for routine complete blood count (CBC), ionogram, and coagulology plus AMH and inhibin B levels were assayed. During standard pre-operative sonographic diagnostic imaging, the number of AFCs was evaluated. Similar laboratory tests and US examination were repeated three months after the surgery. The US was carried out by the same physician.
The laparoscopic cyst enucleation procedure was done by two surgeons. The patients were laid in the Trendelenburg position. Using a Veress needle, pneumoperitoneum was produced. When the intraabdominal pressure attained 14 mm Hg, two 5-mm trocars were inserted via lateral punctures. Then, following ovarian incision, the cyst was enucleated by means of two autraumatic instruments. Some extraordinary situations demanded the use of surgical scissors. The cysts were removed by means of endo bags. Haemostasis by bipolar coagulation required electric power of 40 W, while in the sutured group, ovarian haemostasis was achieved, using Vicryl 2-0 continuous suture.
Results
The pre-operative mean levels of AMH, inhibin B, and AFC did not show statistically significant differences in either group, see Table 1. Also, the observed differences did not attain the level of statistical significance when the same parameters were compared between the sutured group and the coagulated group 3 months after the procedure (Table 2).
Table 1
Comparison of pre-operative AMH, inhibin B, and AFC levels
Table 2
Comparison of post-operative (after 3 months) AMH, inhibin B, and AFC levels
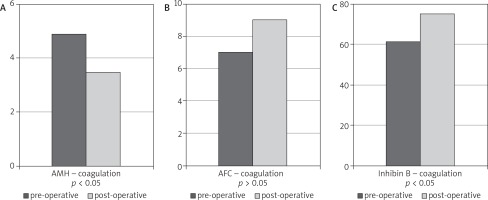
Comparing pre- and post-operative levels of AMH and inhibin B, regardless of the applied haemostasis technique, a statistically significant reduction of the ovarian reserve was observed in either group. In the case of AFC, those differences were not statistically significant, see (Fig. 1, 2).
Fig. 1A-C
The effects of haemostasis technique on ovarian reserve in the group of patients with applied suturing
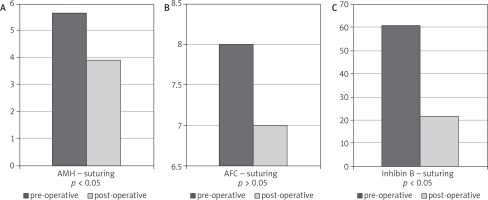
Fig. 2A-C
The effects of haemostasis technique on ovarian reserve in the group of patients with applied electric energy
In further analysis, the effects of haemostasis technique on ovarian reserve were compared in the group of patients with endometrial cysts. The mean AMH level, assayed three months after the surgery, was 2.39 in the patients with ovarian endometriosis and haemostasis, achieved by suturing. Whereas in the patients in whom haemostasis was done by electric energy, the mean AMH level was 4.54. The numbers of antral follicles (AFC) and the levels of inhibin B were also analysed – their differences did not, however, attain statistical significance (Table 3)
Table 3
The effects of haemostasis technique on ovarian reserve in the group of patients with endometrial cysts 3 months after the surgery
| AMH | AFC | Inhibin B | |
|---|---|---|---|
| Ovary coagulation | 4.54 | 2.37 | 21.98 |
| Ovary suturing | 2.39 | 4.28 | 21.82 |
| Statistical significance | p = 0.373 | p = 0.351 | p = 0.912 |
While comparing the ovarian reserve factors among the patients with endometrial and dermoid cysts, the differences in AMH levels were statistically significant in the group where haemostasis was obtained by suturing. However, no statistical significance was observed for AMH values in the group of women with applied bipolar coagulation (Fig. 3B). Table 4 demonstrates the differences between AFC and inhibin B.
Fig. 3A, B
Comparison of AMH values among the patients with endometrial and dermoid cysts, in whom haemostasis was obtained by suturing or coagulation of the ovary
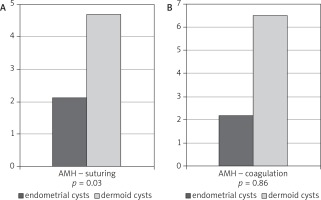
Table 4
Comparison of the ovarian reserve (AFC and Inhibin B) among the patients with endometrial and dermoid cysts, in whom haemostasis was obtained by bipolar coagulation or suturing of the ovary
Discussion
Endometriosis is a chronic disease; therefore, the start of treatment always requires a therapeutic management plan for the whole life of the affected patient, which is associated with the quality of life of the patients with endometriosis. One of the projects trying to cope with this problem was a study by Bergqvist and Theorell [8]. They evaluated the quality of life during hormonal therapy. A group of 48 women participated; the quality of life was assessed by a questionnaire, staging the intensity of symptoms, such as depressive-anxiety disorders, sleep disorders, and quality of life. The authors observed a significantly higher prevalence of depressive-anxiety disorders and sleep problems in women with endometriosis: a six-month therapy with progestogen brought about a considerable regression of the disease. Endometriosis may also significantly affect the sexual life of affected patients. Denny et al. [9] demonstrated that pain sensations during sexual intercourse of patients with diagnosed endometriosis are responsible for limited sexual activity of those women, which in turn leads to downgraded self-esteem and deteriorated relations with the partner.
Regardless of endometriosis progression, laparoscopy is the preferred technique of surgical treatment [10]. Pharmacological treatment may be an element of the patient’s preparation to surgical procedure, while a properly administered pharmacotherapy in postoperative management leads to suppression of further disease recurrence. In this situation, the first-line medicinal agents include: non-steroidal analgesic agents, hormonal bi-component contraceptives, and progestogens. Dienogest is characterised by a high clinical efficacy. Dienogest is a hybrid progestogen because it demonstrates properties that are characteristic for derivatives of both 19-norethisterone and 17-OH progesterone. The results of many studies indicate that dienogest inhibits the angiogenesis and proliferation of endometrial cells, while also suppressing the activity of aromatase and cyclooxygenase-2 (COX-2), and leads to a decreased synthesis of e2 prostaglandin. It means that dienogest not only changes the hormonal profile of treated patients but it also directly influences the endometrial cells, inhibiting their growth and leading to their atrophy [11, 12]. Medicinal agents still in the phase of studies are aromatase inhibitors, selective oestrogen receptor modulators (SERMs), selective progesterone receptor modulators (SPRM), and immunomodulators.
The indications to treatment of endometrial cysts include, first of all, pain, infertility, and next – the risk of malignant transformation. Various techniques of endoscopic treatment of ovarian endometriosis have been reported, including drainage and coagulation, the stripping technique (surgical separation of cyst capsule), or the combined and three-stage technique, proposed by Donnez et al. [13]. The surgical standard in the treatment of endometrial cysts is the enucleation of cystic pseudocapsule. The stripping method – as discussed herein – involves the use of two atraumatic gripping instruments, by which the cyst capsule and the normal ovarian parenchyma are pulled apart in two opposite directions. Some investigators have questioned the laparoscopic technique of endometrial cysts enucleation from the ovary because it poses a risk of removing normal ovarian tissue during cystic capsule separation, leading to the loss of follicles [14, 15]. It was demonstrated in the studies by Donnez et al. that only at the hilar region, the ovarian tissue, removed together with a pseudocapsule, contained primary and secondary follicles [16]. In the other parts of the ovary its tissues, which adhered to the pseudocapsule, did not demonstrate any morphological features of normal ovarian parenchyma – in the majority of cases, either few primary follicles or no follicles were observed. In all the patients qualified to surgery, the technique of ovarian cyst enucleation was the same, i.e. the stripping technique. The only difference was the technique of obtaining haemostasis. It was then possible to compare the effects of ovary suturing and bipolar coagulation on the ovarian reserve.
The ovarian reserve determines the fertility potential of woman: the degree of egg cell resource usage, more accurately, of the primary follicles of which egg cells are formed. A woman is born with a definite pool of egg cells, which are then used up in puberty, adolescence, and then in subsequent monthly cycles until menopause, when they completely disappear. An evaluation of the ovarian reserve enables us to identify to which stage of the process a woman can be assigned. It also makes it possible to assess the current reproductive ability of the ovaries and anticipate a probable reaction of the ovaries to the stimulation of ovulation. The number of AFC (antral follicle count), serum AMH concentration, and serum FSH and inhibin B concentrations, assayed on the second or the third day of the cycle, are the most reliable methods of ovarian reserve evaluation.
AMH is a dimeric glycoprotein, belonging to the group of transforming β growth factors [17, 18]. It occurs both in women and men but plays a different role in each gender. In women, a significant presence of AMH is perceived only after puberty in granule cells of primary ovarian follicles with diameter 4-6 mm, where it is produced [19, 20]. The fact of the exclusively ovarian origin of AMH was confirmed by La Marca et al. in their study, in which AMH levels were undetectable in women after 3-5 days form bilateral ovariectomy [21]. The serum AMH level corresponds to the pool of small ovarian follicles, which is demonstrated by decreased AMH levels in peripheral blood prior to the fall in the number of growing follicles. A constant level of AMH during the menstrual cycle makes AMH a unique endocrine parameter, evaluating the functions of female sex gonads.
The number of antral follicles is the best sonographic determinant of the ovarian reserve. The antral follicles are follicles in the ovaries, of 2-8 mm in diameter, ready to grow under the influence of natural gonadotropins, produced by the pituitary gland, or of the same hormones administered from the outside in therapeutic course. While growing, the antral follicles become dominating follicles and egg cells mature in them. Inhibin B is another marker of the ovarian reserve. It is produced by the granule cells of early antral follicles and released, first of all, during the follicular phase of the menstrual cycle. Its concentration in the early follicular phase reflects the number and quality of ovarian follicles.
In our study, we compared the effects of two haemostasis techniques (ovary suturing and bipolar coagulation) on the ovarian reserve, following laparoscopic cyst enucleation. The mechanism of ovarian reserve reduction is not yet fully recognised. Bipolar coagulation may cause damage to surrounding tissues, as well as ischaemia [22]. Ovary suturing may also lead to ischaemia and reduced ovarian reserve. Another proposed mechanism, which reduces the ovarian reserve, is the development of anti-Mullerian antibodies after laparoscopic cyst enucleation. These antibodies decrease the ovarian reserve, but there are no reports about their post-operative occurrence [23]. A considerable decrease of post-operative AMH levels was observed in a few studies vs. pre-operative AMH values, both in patients with endometrial cysts and in those with non-endometrial cysts; however, the studies concentrated on the first month after laparoscopic surgery [24, 25]. One of the studies demonstrated a big fall in AMH level during the first seven days after surgery, followed by a gradual increase of the anti-Mullerian hormone level, which – after three months – obtained 65% of its baseline value [26]. It was confirmed by the studies of Chang et al. [26], where the ovarian reserve was decreased immediately after cyst enucleation and which gradually returned to its pre-operative value within three months after surgery.
Canis et al. showed that the coefficient of getting pregnant and the number of egg cells, obtained before the procedures of medically assisted reproductive technology, was not significantly decreased after laparoscopic enucleation of ovarian cysts [27]. In turn, Muzii et al. [28] described in their studies an ovarian tissue, adhering to the wall of cystic capsule, in 6% of removed non-endometrial and in 54% of endometrial lesions; however, the adhered and removed tissues did not present the morphological features of normal ovarian tissue.
We found in our study that both ovary suturing and bipolar electrocoagulation, applied after laparoscopic enucleation of cysts, decreased the ovarian reserve. Comparing both haemostasis techniques, no method was demonstrated that would have exerted a smaller impact on AMH, AFC, or inhibin B levels.











