Introduction
The introduction of direct-acting antivirals (DAA) for the treatment of hepatitis C virus (HCV) infections ensured the effectiveness of therapy in patients infected with the dominant genotype 1b HCV at the level of 98%, which even in difficult-to-treat subpopulations exceeds 90% [1-4]. However, despite such excellent treatment effects, there are still patients in whom treatment does not allow for a sustained virologic response (SVR) [5, 6]. In such situations, according to the guidelines, it is reasonable to retry treatment using an alternative therapy [7, 8]. Initially, when treatment failures occurred after genotype-specific DAA regimens, retherapy with alternative drugs from the same group was used. However, soon, the new two-drug pangenotypic therapeutic options glecaprevir/pibrentasvir (GP) and velpatasvir/sofosbuvir (VS) became the natural choice for patients not responding to genotype-specific treatment [6]. Unfortunately, failures also occur among those treated with dual pangenotypic therapies, but the vast majority of them can be successfully re-treated with the use of an alternative dual pangenotypic option. The limitation of this until recently was the possibility of using only one alternative regimen, which was not always possible due to interactions with drugs administered for other indications. This problem was solved mainly by extending the therapeutic options to triple regimens by combining glecaprevir with VS or adding a new protease inhibitor, voxilaprevir, to the VS regimen [5].
The aim of this study was to determine the realworld effectiveness of pangenotypic triple therapy vs. dual drug therapy in treating HCV-infected patients after prior failure of DAA.
Material and methods
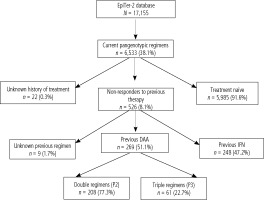
This study included patients from the EpiTer-2 database, which is an ongoing retrospective multicenter national real-world study evaluating antiviral treatment in 17,155 patients with chronic hepatitis C treated in the years 2015-2023. EpiTer-2 is an investigator-initiated study, supported by the Polish Association of Epidemiologists and Infectiologists, which engages 22 Polish centers involved in the diagnosis and treatment of HCV-infected patients. Among patients included in the EpiTer-2 database, there are 6,533 registered patients who received pangenotypic treatment, and in 526 of them, it was retherapy after previous treatment failure. The current analysis included 269 patients treated with either double pangenotypic (P2, n = 208) regimens or triple pangenotypic (P3, n = 61) options as rescue therapy following the previous failure of DAA (Fig. 1).
Antiviral regimens were selected by the attending physician based on current national recommendations [9-14] and the reimbursement policy of the National Health Fund (NHF). The doses and duration of treatment were in accordance with the Summary of Product Characteristics of the individual drugs. All patients gave their informed consent before starting treatment in accordance with the requirements of the NHF. Clinical and laboratory data were collected retrospectively and submitted via an online platform operated by Tiba sp. z o.o. in accordance with the national regulation on the protection of personal data in Poland.
The baseline data included age, gender, body mass index (BMI), comorbidities and concomitant medications, measures of the severity of liver disease, hepatitis B virus (HBV), and human immunodeficiency virus (HIV) coinfections, and the history of previous antiviral treatment. The degree of liver disease was evaluated using transient elastography (TE), shear-wave elastography (SWE), or liver biopsy. The results were transformed to fibrosis stage F0-4 according to the METAVIR score using the recommendations of the European Association for the Study of the Liver (EASL) [8]. Patients with cirrhosis were scored on the Child-Pugh scale and Model of End-Stage Liver Disease (MELD). The HCV RNA was assessed by realtime polymerase chain reaction assays. The intention to-treat (ITT) analysis included patients who received at least one dose of an antiviral drug, and the perprotocol (PP) analysis was established by excluding patients lost to follow-up.
Statistical analysis
The results were expressed as mean (standard deviation) or number (percentage). A p value below 0.05 was considered significant. Fisher’s exact test was chosen to determine whether differences in event frequencies between groups are nonrandom, because the sample size of particular subgroups varied from small (< 10) to large, and in such a case a method based on approximation (i.e., the chi-square test) could not be applied to all analyses. For continuous variables, the Mann-Whitney U test was employed due to the non-Gaussian distribution. Additionally, for the treatment effectiveness analyses, odds ratios (OR) and 95% confidence intervals (CI) were computed. Statistical analyses were performed using the GraphPad Prism 5.1 software (GraphPad Software, Inc., La Jolla, California, United States).
Results
The characteristics of patients presented in Table 1 showed no statistically significant differences between the analyzed groups, except for a significantly more frequent history of liver transplantation in the P3 group (6.6% vs. 0.5%, p = 0.01). Also, in this group, patients with no response to previous therapy were significantly more frequent than in those treated with P2 (41% vs. 26%, p = 0.03) (Table 2). As can be seen from Table 2, patients receiving the P3 regimen significantly more often were previously treated with pangenotypic therapies, and those currently treated with the P2 regimen received primarily genotype-specific therapies. In the P3 group, 12 weeks of voxilaprevir/velpatasvir/sofosbuvir (VVS) therapy was most often used (82%), whereas over 64% of patients receiving the P2 regimen were treated with velpatasvir/sofosbuvir (VS) for 12 or 24 weeks (Table 3).
Table 1
Characteristics of patients receiving rescue pangenotypic triple (P3) or double (P2) therapy following failure of previous direct-acting antiviral regimens.
Table 2
Characteristics of previous regimens and type of non-response.
Table 3
Characteristics of current therapy
Fig. 2
Per protocol end of treatment response (ETR) and sustained virologic response (SVR) in different populations of patients receiving pangenotypic double (P2) and triple (P3) rescue therapies
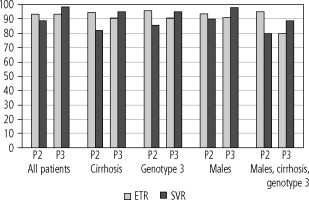
As shown in Table 4, although there were no differences between the two groups in terms of virological response at the end of therapy, the sustained virologic response rate was significantly (p = 0.02) higher in the P3 group, both in the intend-to-treat (95.1% vs. 83.2%), and per protocol after excluding patients lost to follow-up (98.3% vs. 88.7%). The analysis in subgroups of patients traditionally considered difficult to cure showed the same trend, i.e. a similar response rate at the end of treatment but a higher sustained virologic response in the P3 group (Fig. 1). In contrast to the P2 group, the SVR rate following treatment with the P3 regimen was always higher than the end-of-treatment rate (Fig. 1). The differences between the subpopulations were not statistically significant due to the relatively small size of the groups (Table 4).
Table 4
Effectiveness of pangenotypic triple (P3) and double (P2) regimens in all patients and in subpopulations difficult to cure; p-value refers to the Fisher test comparing frequency in both rescue therapy options
In the P3 group, treatment failure was reported in only one male patient infected with HCV genotype 3, with compensated cirrhosis, but with very high liver stiffness (69.1 kPa), treated for 12 weeks with VVS, after previous VS failure, who initially responded at the end of therapy (Table 5). Among the 22 patients from the P2 group, men (59%) and genotype 1b infection (55%) slightly predominated, with 4 HBV and one HIV co-infections. Cirrhosis was diagnosed in 77%, and all but one were classified as Child-Pugh class A and none scored above 14 on the MELD. The vast majority (81%) of patients from the P2 group received VS treatment, and only 4 patients received glecaprevir/pibrentasvir (GP) treatment; 17 patients treated with P2 (77%) failed prior genotype-specific therapy, and only 5 with pangenotypic. In total, as many as 16 patients from the P2 group (73%) who did not achieve SVR had undetectable HCV RNA at the end of therapy (Table 5).
Table 5
Characteristics of patients who failed pangenotypic rescue therapy.
[i] D – detectable, GE – grazoprevir/elbasvir, GP – glecaprevir/pibrentasvir, HBV – hepatitis B virus, HCC – hepatocellular carcinoma, HCV – hepatitis C virus, HIV – human immunodeficiency virus, LS – ledipasvir/sofosbuvir, MELD – Model for End-stage Liver Disease, ND – not done, OPrD – ombitasvir/paritaprevir/ritonavir + dasabuvir, RNA – ribonucleic acid, SR – sofosbuvir + ribavirin, UD – undetectable, UNK – unknown, VS – velpatasvir/sofosbuvir, VVS – voxilaprevir/velpatasvir/sofosbuvir
In the P2 group, the therapy was discontinued in 4 patients, including one due to dyspeptic symptoms, two at the decision of the patients, and one due to death, whereas in P2 all patients completed treatment as scheduled. During treatment, ascites was observed in 2 (1%) and 3 (5%) patients, respectively, and encephalopathy was observed in one patient in each group (0.5% and 1.6%). There was no gastrointestinal bleeding in any of the patients. There were two deaths in the P2 group (1%) and none in the P3 group.
Discussion
With the advent of the era of interferon-free regimens, the percentage of patients with treatment failure has become marginal. However, still a few percent of such patients fail DAA therapy, especially when several negative predictors are present, such as cirrhosis, male gender, or GT3 HCV infection [15, 16]. Lack of response to interferon (IFN)-free treatment is an additional factor associated with a reduced chance of cure, making this population a highly selected, most difficult-to-cure group. The potential impact of resistance-associated substitutions (RAS) cannot be ignored either [17]. Recognizing this difficulty and the importance of the issue, European recommendations clearly indicate that such patients must be managed by experienced treaters and virologists [18]. It should be emphasized that this population is critical to achieving HCV elimination and meeting the WHO goal [19]. Of particular concern are patients after prior treatment with a DAA regimen containing an inhibitor of nonstructural protein 5A (NS5A), who accounted for three-quarters of the population in our analysis. According to international and national recommendations of scientific societies supported by the results of clinical trials, the first-line retreatment option in such patients is the triple combination of DAA, VVS [14, 18, 20-22]. However, in the setting of routine clinical practice, due to limitations in the availability of a triple rescue regimen, double options have been used, especially in patients with risk factors for liver disease progression [6, 23, 24]. This was the approach used in our study, in which the majority of patients with a history of failed DAA therapy received a double pangenotypic regimen for retreatment, with a cure rate of 89%. Two-thirds of these patients received a 12- or 24-week VS combination, while the rest were treated with GP for 8, 12, or 16 weeks depending on the characteristics and according to the labels [25, 26]. Available data from clinical trials involving patients retreated with GP and VS after prior DAA failure have documented SVR rates ranging from 79% to 100%, which is also where our results fall [22, 27-29]. This relatively wide range is due to the fact that the treatment outcome depends on the baseline characteristics of the population in terms of severity of liver disease, HCV genotype, and length of treatment, as well as the combination of these parameters in the analyzed population. The type of DAA regimen previously used is also important; particularly whether it contained an NS5A inhibitor is crucial. This makes simple comparison difficult, especially since the DAA-experienced patient groups included in clinical trials were not numerous. Clinical study data for the retreatment option with the GP regimen come from the MAGELLAN-1 trial evaluating the population infected with GT1 and GT4 [27, 28]. Thirty-three individuals who received a previous regimen containing NS5A inhibitors achieved SVR rates of 88% and 94% at 12- and 16-week treatment durations, while among thirty patients with a history of therapy with inhibitors of HCV protease and NS5A, 79% and 81% responded after 12 and 16 weeks of retreatment, respectively. Higher effectiveness after 16-week GP retherapy was also confirmed in a population of 177 patients after prior SOF + NS5A inhibitor failure; 97% and 95% in patients with and without cirrhosis, compared to 90% and 86% after 12-week therapy [29]. These clinical trial data supported recommendations for the GP regimen as an alternative option for patients with prior failure of SOF + NS5A therapy [20].
Unfortunately, no clinical trial data are available on retreatment with the VS, the second double pangenotypic regimen we analyzed, after failure with a regimen containing an NS5A inhibitor. The only clinical study data on VS retreatment in DAA failures come from the comparative arm of the POLARIS-4 study, where patients who failed regimens without an NS5A inhibitor achieved an SVR of 90% [22]. But based on the analysis of in vitro pharmacology of velpatasvir and the results of treatment of patients with the presence of baseline RASs for NS5A participating in ASTRAL studies, treatment with VS may be considered according to the label in the absence of other available options [26]. The effectiveness of such a strategy was demonstrated in a real-world study by Elhence et al. in a population of 36 patients after the failure of SOF-based options, including those containing NS5A inhibitors [24]. Regardless of baseline characteristics, 100% SVR was documented after excluding patients lost to follow-up. This is a much better result than that demonstrated in our study, but it should be noted that all 5 patients lost to follow-up in the above-cited study were GT3 infected, a subpopulation in our analysis that achieved a lower than overall SVR rate of 85.5%. Other factors that may affect our results are cirrhosis, diagnosed in 14 out of 18 non-responders to VS retherapy, and discontinuation of treatment by two others. In the entire group of 22 patients who did not respond to the pangenotypic double regimens, cirrhosis was diagnosed in 16 patients, and in the subpopulation of cirrhotics only 82% responded to retherapy with the pangenotypic double regimen. After adjusting for gender and genotype, the percentage dropped to even 80% in GT3-infected men. These findings are consistent with those of other RWE analyses showing less effectiveness of DAA therapy in patients with cirrhosis, GT3 infection, and male gender [15, 16, 30]. Moreover, the only patient who did not respond to rescue pangenotypic triple therapy in our analysis was a man with cirrhosis infected with GT3, confirming the negative effect of the combination of these factors on sustained virologic response. After accounting for this one nonresponder, the overall SVR rate achieved with triple pangenotypic retherapy in the analyzed population that, except for two subjects, had been previously treated with an NS5A inhibitor-containing regimen was 98.3%. These results are consistent with data from the POLARIS-1 clinical trial evaluating the 12-week course of VVS, in which an effectiveness of 96% was achieved in such a population; it is worth noting that among the seven nonresponders were two patients after failing VS therapy; both were male, and had cirrhosis and GT3 infection [22]. Therefore, in such patients, it is recommended to add RBV to the 12-week VVS regimen or consider extending the treatment to 24 weeks [20]. Several RWE analyses have also reported lower response rates after retreatment with VVS in GT3-infected patients with cirrhosis, supporting the need to modify the treatment regimen [31, 32]. However, we did not use this approach in our study, similarly to the RWE cohort of 573 US Veterans retreated with VVS without RBV for 12 weeks or less with an SVR of 90.7% [33]. In smaller cohorts from routine clinical practice numbering from 43 to 179 patients, effectiveness ranging from 90% to 100% after retreatment with VVS in NS5A-experienced patients has been reported, depending on patients’ baseline characteristics [31, 32, 34-39]. In contrast, there are very limited data on retherapy with the triple pangenotypic GP-based regimen, recommended for patients after failures with multiple DAA therapies, which in our study was used in seven patients, with a 100% success rate [8, 20, 40].
With a statistically significant difference in virological response in favor of triple regimens documented in our analysis, it is notable that response rates at the end of therapy for the double and triple regimens were comparable, with differences emerging in rates of SVR. Although the trend we observed was not significant in subgroups due to size, it warrants further analysis on larger groups treated with the triple rescue option.
Importantly, in the context of this finding, the patient populations treated with double and triple retherapy did not differ in baseline characteristics, including demographic, laboratory, clinical parameters, and data related to HCV infection, such as genotype and severity of the liver disease. These data are consistent with available clinical trial results reporting a 9.3% relapse rate in the 12-week GP retherapy and 2% for the VVS regimen, but it is worth noting that no relapse was reported in the 16-week GP regimen [22, 27].
Capturing this trend in our analysis was made possible by the determination of HCV RNA at the end of treatment, which is now being phased out to simplify the monitoring of antiviral therapy [7]. The availability of these assessments is one of the strengths of our study. Another strong point is the collection of patients’ data from many centers in the country, treated according to the same recommendations and rules of the drug program, which allows for the generalization of conclusions.
However, we are aware that the research is not free from the limitations related to the real-world design and retrospective data collection, with the possibility of bias, data entry errors, and underreporting of safety data. Additionally, the evaluation of adherence to therapy was not assessed by an objective method; we relied only on patients’ declarations. Finally, we did not test the presence of RAS at baseline since it is not required in the Polish drug program. According to the recommendations of scientific societies, RAS testing may optimize the management of patients after failure of DAA therapy; however, available data indicate no significant impact of RAS on the effectiveness of rescue pangenotypic retherapy [41].
Conclusions
A comparison of double and triple pangenotypic retherapy in patients after failure of DAA therapy showed a higher sustained virological response in the triple option with a comparable response at the end of therapy. The factors reducing the chances of cure were cirrhosis, genotype 3 infection and male gender. However, due to the small size of the subgroups, no significant differences were found, which justifies further studies in patients treated with rescue triple regimens.