Introduction
Meigs syndrome is a rare gynecological condition characterized by the presence of a benign ovarian solid tumor accompanied by ascites and hydrothorax. Rarely it is associated with high levels of CA 125. Meigs syndrome is more common in postmenopausal women with an average age of about 50 years. Even though it is extremely rare in women aged less than 30 years, some cases have been reported in children aged 4 and 9 years. Surgery is necessary to confirm the diagnosis. Histologically, the majority of cases are represented by fibroma, but thecoma, cystadenoma and granulosa cell tumor are also described. The typical features of the syndrome, ascites and pleural effusion, resolved completely after the surgical removal of the tumor. Although it mimics a malignant condition, Meigs syndrome is a benign disease [1, 2], characterized by a good prognosis.
Case report
A Caucasian 62-year-old woman referred to our department due to an abdominal pain since a few days before, associated with nausea and vomiting. She also presented with dysphagia and cachexia. No hirsutism, skin discoloration or other signs of hormonal dysregulation were detected. A history of painless, progressive abdominal distension and increasing weight of more than 15 kg in one year were reported, reaching a body mass index of 30.5. Her anamnesis was substantially silent and she did not have familiarity for gynecological cancer or pathology. At admission a very distended abdomen was detected upon examination, while massive ascites was observed at the office ultrasound (US) scan. During hospitalization the patient complained of progressive dyspnea, especially in supine decubitus.
Investigations
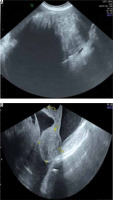
In the suspicion of a malignant ovarian mass, diagnostic investigations were performed. Ovarian markers showed increased levels of CA 125 (1744.3 mUI/ml), with normal levels of carcinoembryonic antigen (4.2 ng/ml), AFP (3.5 ng/ml) and Ca 19.9 (19.2 UI/ml). The office transabdominal (TA) US showed the presence of massive ascites. A second level US (transvaginal and TA) revealed a 20 cm in diameter mobile solid mass with smooth surface with color score 2 and acoustic shadows in the right adnexal region [3]. There was no sign of pelvic structures’ infiltration or abdominal carcinomatosis (Fig. 1 A). The uterus was within the normal range and a small fibroma was described.
Fig. 1
A – Ultrasound (US) transabdominal image showing the right adnexal mass and acoustic shadows, B – US transvaginal image showing massive ascites in the pouch of Douglas and a normal uterus
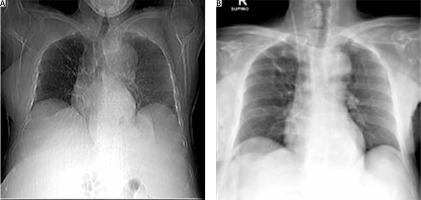
Thoracic and abdominal computed tomography (CT) scans were performed for a better assessment. They revealed conspicuous peritoneal effusion and an abdominal mass of about 17 cm of doubtful origin. A non-homogeneous uterine neoformation of about 43 mm was described and interpreted as a fibroleiomyoma. A chest and chest CT scan confirmed a modest level of monolateral left hydrothorax (Fig. 1 B).
Differential diagnosis
The combination of ascites, pelvic mass and high levels of CA 125 raised suspicion of a potential ovarian malignancy. The differential diagnosis for the presenting signs and symptoms included malignant ovarian tumor, other bowel or lung cancers, nephrotic syndrome, congestive cardiac failure, liver cirrhosis and tuberculosis [4]. This disease represents a diagnostic and therapeutic challenge for the gynecologist.
Treatment
Due to the progressive dyspnea, the patient was submitted urgently to a preliminary laparoscopic exploration at the subsequent laparotomic access (xypho-pubic or navel-pubic laparotomy) and the radical nature of the intervention. During laparoscopy we performed multiple biopsies and the aspiration of 20 liters of ascitic fluid. Laparoscopic exploration showed stomach, liver, peritoneum, bowel and omentum free of disease. In the right iliac fossa a 20 cm capsulated solid polylobate smooth neoformation was found. The patient was then submitted to a laparotomic hysterectomy, bilateral adnexectomy, asportation of the pelvic mass and multiple biopsies. There was no pelvic or aortic lymphadenomegaly. Histological examination diagnosed an ovarian fibrothecoma with large fibrohyaline areas. The cytologic examination of ascites and hydrothorax did not show any cells suggestive of malignancy.
Outcome and follow-up
The postoperative period was characterized by complete resolution of hydrothorax (Fig. 2 A, B) and ascites with reduced levels of CA 125 (227.1 mUI/ml) at only 10 days after surgery. CA 125 was within the normal range at one month later. The patient underwent gynecological follow-up examination and US at 1 and 6 months after surgery. Both follow-ups were negative. The patient was asymptomatic and in good health.
Discussion
Meigs syndrome is characterized by the presence of a benign ovarian solid tumor accompanied by ascites and hydrothorax. Rarely this condition could be associated with high CA 125 levels. Although it mimics a malignant condition, Meigs syndrome is a benign disease [1, 2, 5].
The postmenopausal findings of ascites, solid monoor bilateral adnexal mass, pleural effusion and elevated serum CA 125 are highly suggestive for malignant ovarian tumor. In fact, CA 125 is increased in 80% of advanced epithelial ovarian cancers [6, 7]. Nevertheless, it could be raised during menstruation or pregnancy and in some benign conditions such as endometriosis, peritonitis or cirrhosis, particularly with ascites. Probably in this case its elevation is due to inflammation and secretion from mesothelium cells, and it also happens in pseudo-Meigs syndrome [8, 9].
This disease is usually characterized by the presence of an ovarian fibroma or a fibrothecoma, which is rarely associated with increased levels of tumor markers [10]. Ovarian fibroma is a benign tumor, which accounts for approximately 3% of all ovarian tumors and may be pure and non-secreting. Meigs syndrome occurs in 1–10% of cases associated with this tumor. Ovarian fibroma is the most frequently observed in this syndrome, at the rate of 80–85% [10]. Sometimes thecoma elements (fibrothecomas) are present and responsible for estrogen secretion [11]. Thecomas and fibrothecomas represent 10% of tumors associated with Meigs syndrome. Fibrothecoma is a benign ovarian stromal tumor, usually seen as a unilateral lesion [10]. In contrast, pseudo-Meigs syndrome is characterized by ascites and pleural fluid secondary to other pelvic or abdominal tumors. This condition was further subclassified into 2 categories: benign pseudo-Meigs syndrome and malignant pseudo-Meigs syndrome. The first term was used for patients with symptoms related to any benign pelvic or abdominal tumors located outside the ovaries, fallopian tubes, and broad ligaments, whereas the second refers to patients with malignant pelvic or abdominal tumors (primary or metastatic) [12]. In Meigs syndrome, by definition, peritoneal or pleural spread of the tumor must be excluded (negative pleural and peritoneal fluid cytology and/or no malignant involvement in biopsy samples) and both ascites and hydrothorax should resolve after tumor removal [13].
The pathogenesis of the disease is unknown. The production of ascitic fluid in Meigs syndrome could be due to an imbalance between vascular supply and lymphatic drainage. An alternative hypothesis is the inflammatory origin of the process, with elevation of several inflammatory molecules including cytokines, vascular endothelial growth factor, fibroblast growth factor, interleukin (IL)-1b, IL-6, and IL-8. The detailed underlying mechanism is still unclear. Furthermore, the etiology of pleural effusion is uncertain. It is probably due to the passage of ascitic fluid into the pleural space through the diaphragm or diaphragmatic lymphatic vessels, which are more common on the right side [13, 14]. Indeed, in Meigs syndrome the hydrothorax is mainly unilateral and occurs most often on the right side (75%), rarely on the left side, and sometimes it is bilateral. The size of the pleural effusion is largely independent of the amount of ascites [14].
The general condition of the patient can be of variable severity. The classical triad (ascites, abdominal mass, and hydrothorax) may be a chance discovery during a routine gynecological examination or become symptomatic, causing abdominal tension with bloating and weight gain, respiratory distress associated with cough or abdominal pain secondary to adnexal torsion. In the literature, most patients present with an ovarian asymptomatic large, solid, unilateral mass, mostly left-sided.
Meigs syndrome always requires surgical treatment. A prompt differential diagnosis between benignity and malignancy has to be made in order to choose the appropriate management.
Ovarian tumors are common in women of all ages. It has been estimated that in the female population, the lifetime risk of undergoing surgery for a suspected ovarian neoplasm is 5–10% [15]. However, the incidence of ovarian cancer is low, even though it represents the most lethal gynecological malignancy [15]. In order to ensure that ovarian cancer patients access appropriate treatment to improve the outcome of this disease, accurate characterization before any surgery on ovarian pathology is essential. For this reason, the work of the International Ovarian Tumor Analysis (IOTA) collaboration in standardizing terminology, definitions and characteristics that must be described in adnexal pathology is critical [3]. Being based on diagnostic algorithms, the group developed and validated risk prediction models (simple rules, LR1, LR2, Adnex) on a large sample of patients; these are highly diagnostic and therefore superseded previous algorithms. Even if the assessment according to IOTA rules by an experienced examiner is subjective, it is still widely considered as the most accurate method for classification of preoperative adnexal masses. IOTA risk prediction models showed a high predictive value compared to other, non-IOTA algorithms [16]. The “simple rules” have been shown to apply to over 75% of masses and have been successfully externally validated and implemented in a national protocol [17, 18]. Nevertheless, in our case, the analysis according to IOTA simple rules revealed “inconclusive results”; the logistic regression model LR2 and Adnex [17] suggested a high risk of malignancy. In fact, the presence of ascites and the size of the lesion affected the correct assessment of the risk of malignancy because these are usually characteristics of malignant ovarian cancer. It is also reasonable and evidence-based to believe that the lesion size affects diagnostic performance of IOTA prediction models in discriminating between malignant and benign ovarian pathology [19].
Moreover, the prevalence of different types of histology affects the performance of subjective assessment in correctly classifying adnexal masses as benign or malignant [20, 21]. In this patient, the “subjective assessment” according to IOTA rules by an expert US examiner [16] was used to understand the nature of the mass. This ovarian mass was very large, solid and accompanied by ascites, but presented acoustic shadowing, a smooth external surface, mobility and a low color score (color score 2 according to IOTA terms) [3]. The mass could seem benign, as it was. Type I ovarian tumors (low-grade serous, low-grade endometrioid, clear cell, and mucinous) are slow growing, reaching a large size while still confined to the ovary, and are thus likely to be detected early by transvaginal US. Unfortunately, these lesions constitute only 25% of ovarian cancers and account for only approximately 10% of ovarian cancer deaths. On the other hand, type II ovarian tumors (high-grade serous and undifferentiated carcinomas and carcinosarcomas) represent 75% of all ovarian carcinomas and are responsible for 90% of ovarian cancer deaths and may originate outside the ovary. These tumors are almost never confined to the ovary at first evaluation, making an early diagnosis still a challenge [16]. Usually, the CT scan does not diagnose with certainty the origin of the masses. A diagnostic laparoscopy is useful in patients with potentially malignant tumors, and the tumor resection can also be performed if there are signs of malignancy [22, 23]. Depending on the patient’s age, a unilateral salpingo-oophorectomy is
commonly performed for the treatment of an ovarian type I tumor. For women who desire
preservation of the ovary, an ovarian cystectomy may be performed with complete excision of the fibromatous tissue [24, 25]. However, the optimal approach for its management has still not been sufficiently investigated [26]. Meigs syndrome is curable by tumor resection and should be differentiated from malignancy. The connection between the pelvic tumor and ascites is confirmed by the rapid resolution of symptoms due to the complete disappearance of ascites and/or the fluid in the pleura following the surgical removal of the tumor with a complete “restitutio ad integrum”. The prognosis of Meigs syndrome is favorable, recurrence of peritoneal and pleural fluid after complete removal of the tumor is unlikely to occur, and life expectancy after surgical removal is the same as in the general population [2]. So, fast surgical management of the patients is mandatory. The main limitation of our management is that our therapeutic approach has been radical despite the presence of the classical triad of symptoms suggestive for Meigs syndrome. Otherwise, the main strength of our management is that all clinical, instrumental and histological findings were essential to formulate the correct diagnosis, ensuring the complete regression of the patient’s symptoms. The major message related to this case report is that abdominal tumor, ascites, pleural effusion and elevated CA 125 – symptoms strongly suggesting disseminated malignancy – do not necessarily mean advanced malignant disease.
Conclusions
Clinicians should consider the uncommon Meigs syndrome in the case of a pelvic mass suggestive for an ovarian fibroma/fibrothecoma at US examination, ascites and hydrothorax, especially in the case of low levels of CA 125. Meigs syndrome is often misdiagnosed as a malignant condition in the presence of high levels of CA 125. Life expectancy and prognosis of Meigs syndrome are favorable with the complete “restitutio ad integrum” after surgical removal of the pelvic mass and the subsequent hydrothorax and ascites resolution.