Introduction
Reconstruction of the right ventricular outflow tract (RVOT) is one of the most common surgical procedures for the treatment of complex congenital heart defects. Despite significant advances in surgical techniques and the availability of various materials used for RVOT reconstruction, these conduits degenerate over time, making repeated surgical treatment necessary. Early reports presented promising results of a new, fully synthetic, bioabsorbable graft [1].
We report the first-in-man percutaneous pulmonary valve implantation (PPVI) in a failed novel Xeltis pulmonary valve conduit (XPV; Xeltis, Eindhoven, The Netherlands).
Case report
A 14-year-old girl, born with tetralogy of Fallot (TOF), underwent staged treatment with a systemic-to-pulmonary artery shunt and total repair with a transannular patch. The child’s growth was rated as good, but with progressively worse exercise tolerance. At the age of 8 years, during transthoracic echocardiography, an enlarged right ventricle and significant pulmonary regurgitation were noted. Subsequent cardiac magnetic resonance (CMR) revealed indexed right ventricular end diastolic volume of 146 ml/m2 and severe pulmonary valve regurgitation of 43%.
The patient underwent reconstruction of the RVOT using the first generation of the 18 mm diameter Xeltis pulmonary valve conduit (XPV-1) (Figures 1 A–E). The surgical procedure and postoperative course were uneventful. Follow-up echocardiography showed mild right ventricular enlargement with normal systolic function, tricuspid regurgitation with a maximum gradient of 18 mm Hg, and a trace of pulmonary regurgitation with pulmonary flow of 2 m/s (Figures 1 F, G).
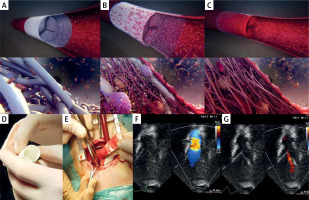
Figure 1
A–C – Artistic vision of endogenous tissue restoration process after implantation of the Xeltis Pulmonary Valve conduit (XPV; Xeltis, Eindhoven, The Netherlands) implanted into the human heart (source: https://xeltis.com/). D – Xeltis pulmonary valve conduit prepared for implantation. Visible leaflets of valve. E – Implantation of the Xeltis pulmonary valve conduit through a 5 cm ministernotomy. F, G – Echocardiographic visualization 3 months after Xeltis implantation – unobstructed flow with a trace of regurgitation
In the first year of follow-up a gradual increase of regurgitation of the XPV-1 graft was noted. In the fourth year after the implantation, an enlarged right ventricle, significant graft regurgitation and increasing stenosis of the distal (supra-valvar) part of the graft, with a maximum gradient of 90 mm Hg, were found. The reason for the failure of the graft was some kind of kinking of the stiff graft with subsequent unfavorable remodeling. Repeat CMR revealed indexed right ventricular end diastolic volume of 141 ml/m2, graft regurgitation fraction of 19% and flow acceleration through the graft corresponding to a maximum gradient of 48 mm Hg. The patient was scheduled for PPVI.
During cardiac catheterization haemodynamic measurements showed: right ventricular pressure of 58/11 mm Hg, which was 78% of the systemic pressure (left ventricular pressure 74/7 mm Hg) and normal pulmonary artery pressures (18/7/12 mm Hg). Angiography detected dilated proximal RVOT with significant multilevel narrowing of the graft to the minimum diameter of 10–12 mm (Figure 2 A). With the use of a low-pressure 18 × 40 mm MaxiLD balloon (Cordis) inflated in the RVOT and concomitant aortography, coronary compression was excluded, and sufficient distance between the graft and the coronary arteries was confirmed, even with the need to use a stent larger than the size of the measuring balloon. Next, a 34 mm covered Cheatham Platinum (cCP) stent (NuMed) was selected to prepare the landing zone for a pulmonary valve. The cCP stent was mounted on a 20 × 45 mm BiB catheter (NuMed) and advanced into the XPV-1 through a long 14 Fr Mullins sheath (NuMed). During the passage of the stent through multilevel narrowings of the graft, the stent partially slipped distally off the delivery balloon. It was not possible to pull the stent/balloon assembly back to the long sheath; hence the stent was intentionally pushed further distally off the balloon to the distal right pulmonary artery. Securing the guidewire position, the BiB catheter was replaced with an 18 × 40 mm Tyshak II balloon (NuMed) (Figure 2 B), which was slightly inflated in the stent parked in the distal right pulmonary artery. Subsequently the stent was repositioned (Figure 2 C) and deployed in the graft (Figure 2 D). Then the stent was further dilated with a high pressure 20 × 40 mm Atlas Gold balloon (Bard Peripheral Vascular) (Figure 2 E).
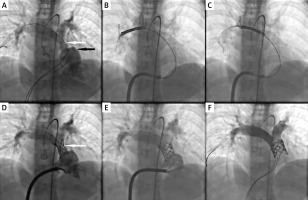
Figure 2
Step-by-step angiographic images of percutaneous pulmonary valve implantation into the narrowed first generation Xeltis Pulmonary Valve (XPV-1) conduit. A – Dilatation of proximal right ventricular outflow track (black arrow) and significant multilevel narrowing in the middle and distal part of the graft (white arrow). B – Non-expanded stent in the distal part of the right pulmonary artery branch with slightly inflated low pressure catheter balloon inside (black empty arrows). C – Stent repositioning on the slightly inflated low pressure catheter balloon (black empty arrows) to the desired position in the graft. D – Control angiography after stent implantation on the low pressure 18 mm catheter balloon in the expected position showed persistent narrowing. E – Control angiography after stent dilatation with a high pressure 20 mm catheter balloon showed total reduction of the narrowing. F – Final angiography after implantation of 22 mm valve and post-dilation with 22 mm high pressure balloon showed no pulmonary regurgitation or narrowing
Finally, a 22 mm Melody valve (Medtronic Inc) was delivered on a 20 mm Ensemble delivery system to the landing zone. The whole assembly was then dilated with a 22 × 40 Atlas Gold balloon up to 16 atm. The final angiography showed a uniformly deployed cCP stent and the Melody valve in the XPV-1, without any ruptures, or contrast extravasations (Figure 2 F). Haemodynamic measurements confirmed a significant decrease in the right ventricle pressure (RV 25/5 mm Hg; aorta = 88/66/77 mm Hg, RV/Ao = 28%) and significant RVOT gradient reduction from 40 mm Hg to 5 mm Hg. The recovery was uneventful. Discharge echocardiography showed good function of the moderately enlarged right ventricle, trivial central regurgitation of the Melody valve with a maximum gradient through the valve of 19 mm Hg. In the 12-month follow-up, further decrease in right ventricle size was noted with maximum gradient through the Melody valve of 10 mm Hg and mild regurgitation. In repeated fluoroscopy evaluations, the stent and valve showed no signs of fracture or distortion.
Discussion
Refinements in surgical techniques for treatment of TOF have led to low early morbidity and mortality [2]. However, with increasing time from the ‘corrective’ surgery the rate of reinterventions significantly increases [3]. Patients treated with a transannular patch typically present with increasing pulmonary regurgitation and those with a valved conduit or bioprosthesis experience progressive RVOT dysfunction, either stenosis, regurgitation or a combination. Both groups will require several surgical or transcatheter interventions during their lifetime.
Percutaneous pulmonary valve implantation enables reduction of the number of surgical conduit replacements [4]. There are many reports presenting excellent results of PPVI in various anatomies and after various surgical treatments [5–7]. New valve designs, modifications of percutaneous implantation techniques, and advanced imaging and a hybrid approach have further widened the group of patients who can benefit from a trans-catheter valve [8–12].
Recently, in the ongoing pursuit for the ideal conduit, interest has been focused on tissue and genetic engineering [13]. Biorestorative materials have brought hopes for conduit growth, improved biocompatibility, and subsequently longer durability and unlimited availability of various sizes. A novel pulmonary valved conduit – XPV – received favorable reports in studies that examined sheep and humans [14, 15]. This fully synthetic, seamless and highly porous conduit is based on a polymer platform. As the graft progressively resorbs, all components of the native tissue invade the graft and leaflets; they develop and become organized into a natural tissue [1, 15]. The technique of XPV implantation is similar to any pulmonary graft implantation; however, due to some stiffness of the material, efforts are needed to preserve the cylindrical shape of the XPV. As we currently know, any deformities may result in valve insufficiency and improper remodeling of the graft with potential narrowings. In the first generation XPV-1, like in our patient, progressive regurgitation of the graft was observed. This issue was addressed in the second generation (XPV-2), where the mechanical properties of the conduit (including leaflets) have been redesigned. Gaining experience with the dedicated technique of implantation of the novel graft helps to eliminate any deformities and subsequent unintended remodeling of shape.
In our patient, 5 years after implantation of the XPV-1, re-intervention was necessary due to mixed conduit dysfunction. The question remained whether to use a surgical or percutaneous approach. The mechanical properties of the XPV-1 after a 5-year follow-up in humans were uncertain, making PPVI unpredictable. On the other hand, the patient had already undergone three operations including two sternotomies and cardiopulmonary bypass runs. With a surgical team on standby, PPVI was attempted. A low pressure, slightly undersized balloon was used for exclusion of potential coronary artery compression. Similarly, the balloon for delivery of the pre-stent and the valve delivery system were smaller in diameter than the planned final diameter of the valve to reduce the risk of conduit fracture. After securing the landing zone with a covered stent and adding another covering with the Melody valve, the entire assembly was dilated with high pressure to the desired diameter. The necessity of using very high pressure for dilation of the graft confirms strong mechanical properties of the novel restorative graft 5 years after implantation, once the process of restoration is definitely completed.
Conclusions
We report the first PPVI into a novel restorative pulmonary valve conduit. Low pressure balloon testing of coronary artery compression, selection of a covered stent for the valve landing zone and high pressure post-dilatation of the stent/valve assembly provided safe and effective relief of RVOT obstruction and restoration of valve function.








