Summary
Elderly patients with severe aortic valve stenosis subjected to surgical (AVR) and transcatheter aortic valve implantation (TAVI) are exposed to increased risk of bleeding. Although procedure-related hemostasis disorders might be crucial for safety of both procedures, the amount of data is negligible. We performed peri-procedural analyses of global hemostasis and platelet reactivity in 30 patients ≥ 70 years old subjected to AVR and TAVI. The surgical procedure was characterized by quickly recovered hypofibrinogenemia and von Willebrand factor depletion. Since TAVI was mainly characterized by platelet dysfunction with increased sensitivity to antiplatelet agents, early dual antiplatelet prophylaxis after TAVI requires careful consideration.
Introduction
Severe degenerative aortic valve stenosis (AS) induces multidirectional hemostasis disorders [1–6]. The most investigated are acquired von Willebrand (vWF) syndrome with multimers depletion and enhanced platelet activation associated with high shear forces and ongoing inflammation in aortic valve tissue [1–6]. The only proven methods of AS treatment are surgical aortic valve replacement (AVR) and transcatheter aortic valve implantation (TAVI) [7]. Despite the high effectiveness of these procedures, both approaches are invariably burdened with a risk of serious, procedure-related bleeding [7–11]. Procedure-related hemostasis disorders that overlap on initial coagulopathies might be crucial for the safety of both interventions. Yet, the amount of data on the peri-procedural status of hemostasis in patients subjected to AVR and TAVI is negligible [3, 12–22].
Aim
Therefore, the main aim of our study was to investigate the profile of peri-procedural hemostasis in elderly patients with AS, subjected to AVR and TAVI. Despite the obvious differences in both methods of treatment, we additionally compare hemostasis disorders associated with the procedures.
Material and methods
We carried out a prospective, single-center, nonrandomized, explorative and hypothesis-generating study with 30 patients subjected to aortic valve implantation. All consecutive patients scheduled for elective isolated TAVI or AVR between March 2017 and October 2017 were screened for eligibility. Inclusion and exclusion criteria are described in Table I. The decision about the type of aortic valve procedure was based on the current guidelines, and was reached by our heart team consisting of an interventional cardiologist and a cardiac surgeon [7].
Table I
Inclusion and exclusion criteria
Procedures and peri-procedural anticoagulation
All procedures were performed in the 1st Department of Cardiac Surgery, American Heart of Poland Inc., in Bielsko-Biala, Poland, under general anesthesia. All TAVI procedures were performed with surgical access via the femoral artery, and the use of CoreValve Medtronic prostheses. All AVR were performed with median sternotomy followed by cannulation of the aorta and the right atrium access for extracorporeal circulation, using Medtronic Hancock II, Medtronic Mosaic, and St Jude Epic prostheses.
The anticoagulation protocol for AVR and TAVI was based on unfractionated heparin (UFH). During the TAVI procedure 70–100 IU/kg body weight of UFH was administered with a target activated clotting time (ACT) of 250–300 s. In the case of AVR, 1.0–2.0 g of tranexamic acid was administered intravenously before the procedure. The dose of UFH was 350–400 IU/kg body weight to achieve ACT of 350–400 s. After procedures UFH action was reversed by protamine sulfate in a 0.8–1 : 1 ratio.
All patients received aspirin prophylaxis. Patients on chronic aspirin prophylaxis received 75 mg o.d. of drug until the day of the procedure. Aspirin naïve patients received a loading dose of 300 mg of aspirin on the day preceding interventions. Starting from day of the procedures, long-term aspirin prophylaxis was recommended. After TAVI 75 mg o.d. of aspirin, and after AVR 150 mg o.d. of aspirin was recommended. During the first 2 post-procedural days 40 mg o.d. of low molecular weight heparin was administered subcutaneously in all patients. In TAVI patients, clopidogrel prophylaxis with 75 mg o.d. was started not earlier than 24 h after the procedure, only if proper hemostasis was achieved. In case of bleeding complications clopidogrel administration was postponed until the recovery of proper hemostasis.
Hemostasis analyses
All tests were performed at 3 time points: within 24 h before, directly after (30 min–1 h after protamine administration), and 24 h after procedures (before clopidogrel administration in TAVI patients).
Global hemostasis including intrinsic, extrinsic coagulation pathways, platelets and fibrinogen activity were assessed by thromboelastometry, with the ROTEM delta device (Tem International GmbH, Germany), using rexTEM (thromboplastin, extrinsic coagulation pathway and platelet activity), inTEM (ellagic acid, intrinsic coagulation pathway), fibTEM (cytochalasin D – platelet inhibitor, fibrinogen concentration and fibrin polymerization ability), hepTEM (heparinase – heparin inactivation, and intrinsic coagulation pathway), and starTEM tests (calcium chloride, recalcination of citrated blood). The main parameters which reflect hemostasis are: coagulation time (CT) – time from the beginning of the test until the clot reaches 2 mm of amplitude – activation of plasma coagulation factors and platelets; clot formation time (CFT) – time when clot firmness is increasing from 2 mm to 20 mm – the effectiveness of primary and secondary hemostasis to form the clot; maximum clot firmness (MCF), maximum clot size – overall hemostatic potential; amplitude time (Ax) – clot firmness at the respective time points after CFT; maximum lysis (ML), the percentage of clot reduction in comparison to MCF – maximal lysis detected during the run time.
Platelet function was assessed using impedance aggregometry, Multiplate analyzer (Roche Diagnostics Ltd., Switzerland), and ADPtest, ASPItest, TRAPtest, RISTOtest. Platelet reactivity in ASPItest and ADPtest reflects the sensitivity to antiplatelet agents – aspirin and inhibitors of P2Y12 receptors. TRAPtest and RISTOtest expressed ability of platelets to activate in response to potent platelet activators – thrombin and vWF. Results of RISTOtest indirectly reflect the vWF activity.
The operating principles of the Multiplate and ROTEM delta analyzer have been described elsewhere [16, 17, 23, 24].
Additionally, standard blood count tests with the assessment of hemoglobin concentration – Hgb (g/dl), platelet count – PLT (1000/l), international normalized ration (INR) and activated partially thromboplastin time (APTT) were performed at the same time points.
Bleeding associated with TAVI and AVR was defined according to the Valve Academic Research Consortium 2 definition, including major and life-threatening/disabling bleeding (Table II).
Table II
Clinical characteristics of study population and procedural outcomes
| Clinical characteristics | AVR (n = 15) | TAVI (n = 15) | P-value |
|---|---|---|---|
| Age, mean ± SD [years] | 74.93 ±5.78 | 78.13 ±4.38 | 0.13 |
| EuroSCORE II %, mean ± SD | 6.45 ±1.41 | 11.48 ±4.14 | < 0.01 |
| Sex – female, n (%) | 11 (73.33) | 8 (53.33) | 0.88 |
| Hypertension, n (%) | 15 (100) | 15 (100) | – |
| Diabetes mellitus, n (%) | 8 (53.33) | 7 (46.66) | 0.78 |
| Coronary artery disease, n (%) | 8 (53.33) | 10 (66.66) | 0.07 |
| Previous PCI, n (%) | 2 (13.33) | 9 (60) | 0.23 |
| Previous CABG, n (%) | 0 | 3 (20) | 0.34 |
| Heart failure, n (%) | 9 (60) | 14 (93.33) | 0.41 |
| NYHA class, n (%): | |||
| I | 1 (6.66) | 0 | |
| II | 12 (80) | 6 (40) | 0.26 |
| III | 1 (6.66) | 9 (60) | |
| IV | 1 (6.66) | 0 | |
| Renal failure, n (%) | 4 (26.66) | 11 (73.33) | 0.93 |
| Stroke/TIA, n (%) | 1 (6.66) | 3 (20) | 0.69 |
| COPD, n (%) | 1 (6.66) | 2 (13.33) | 0.69 |
| Peripheral arterial disease, n (%) | 1 (6.66) | 7 (46.66) | 0.28 |
| Liver dysfunction, n (%) | 1 (6.66) | 1 (6.66) | 0.78 |
| Atrial fibrillation, n (%) | 6 (40) | 7 (46.66) | 0.61 |
| Peri-procedural parameters and in-hospital outcomes, mean ± SD: | |||
| LVEF before procedure (%) | 55.86 ±4.61 | 50.6 ±5.27 | 0.005 |
| PGmax before procedure [mm Hg] | 89.46 ±16.76 | 92.4 ±20.59 | 0.68 |
| PGmean before procedure [mm Hg] | 51.6 ±12.12 | 62.0 ±17.08 | 0.09 |
| Vmax before procedure [m/s] | 4.8 ±0.5 | 4.47 ±0.32 | 0.03 |
| PGmax after procedure [mm Hg] | 30.53 ±10.64 | 24.33 ±3.28 | 0.06 |
| PGmean after procedure [mm Hg] | 15.13±7.56 | 11.46 ±1.72 | 0.09 |
| Vmax after procedure [m/s] | 2.71 ±0.62 | 2.36 ±0.15 | 0.13 |
| Aortic regurgitation ≥ moderate, n (%) | 0 | 0 | – |
| Anemia before procedure | 5 (33.33) | 7 (46.66) | 0.07 |
| Hgb before procedure | 12.95 ±1.63 | 12.07 ±1.00 | 0.08 |
| Hgb directly after procedure | 10.22 ±1.16 | 10.82 ±1.04 | 0.2 |
| Hgb 24 h after procedure | 11.15 ±0.64 | 10.9 ±1.14 | 0.32 |
| PLT before procedure | 218.33 ±29.9 | 190.73 ±47.88 | 0.07 |
| PLT directly after procedure | 139.4 ±28.91 | 141.8 ±40.18 | 0.86 |
| PLT 24 h after procedure | 163.2 ±17.64 | 140.6 ±39.79 | 0.08 |
| APTT before procedure | 29.06 ±2.01 | 32.32 ±4.16 | 0.01 |
| APTT 24 h after procedure | 41.30 ±6.21 | 35.64 ±5.77 | 0.03 |
| INR before procedure | 0.96 ±0.06 | 1.21 ±0.63 | 0.15 |
| INR 24 h after procedure | 1.17 ±0.65 | 1.08 ±0.24 | 0.21 |
| Drainage [ml]: | |||
| 12 h after AVR | 315.33 ±150.04 | – | |
| In total | 447 ±161.29 | ||
| Reoperation due to bleeding, n (%) | 2 (13.33) | 3 (20) | 0.46 |
| Bleeding, n (%)* | 3 (20) | 6 (40) | 0.3 |
| Transfusion, n (%): | |||
| PRBC | 4 (26.66) | 7 (46.66) | 0.88 |
| FFP | 3 (20) | 1 (6.66) | 0.04 |
APTT – activated partial thromboplastin time, AVR – aortic valve replacement, CABG – coronary artery bypass grafting, COPD – chronic obstructive pulmonary disease, FFP – fresh frozen plasma, Hgb – hemoglobin, INR – international normalized ratio, LVEF – left ventricle ejection fraction, PCI – percutaneous coronary intervention, PG – transvalvular pressure gradient, PLT – platelet count, PRBC – packed red blood cells, TIA – transient ischemic attack.
Ethics
The study was performed in compliance with the Declaration of Helsinki, and was approved by the Local Ethics Committee. Written informed consent was obtained from all individuals.
Statistical analysis
Continuous variables were presented as means and standard deviations or medians and interquartile range for Gaussian and non-Gaussian distribution of the variable respectively. In statistical analyses the parametric t-test or nonparametric Mann-Whitney U test was performed for normal and non-normal variables respectively. Multivariate analysis of variance (MANOVA) with post hoc analysis was performed for the multivariate analyses. The value p < 0.05 was considered statistically significant. All statistical analyses were performed using Statistica 12.0 software (StatSoft, Inc. 2014. Statistica, version 12).
Results
We enrolled 30 patients, of whom 15 underwent AVR, and 15 were subjected to TAVI. Patients who underwent TAVI had significantly higher surgical risk, due to the greater number of comorbidities (Table II). Left ventricle ejection fraction and related with it maximal transvalvular velocity were higher in patients subjected to AVR. Otherwise, the study population was well balanced in terms of clinical characteristics.
Thromboelastometry
Patients subjected to AVR had significant hemostasis disorders related to the procedure. In comparison to initial parameters, directly after the procedure external pathway initiated hemostasis was characterized by prolonged activation of plasma factors and platelets (CT; p = 0.04), prolonged clot formation (CFT; p = 0.01) and reduced clot firmness at each time point (A5-A25, MCF; p = 0.03). Within the next 24 h after AVR all hemostasis parameters were significantly improved, reaching values similar to the initial ones. Comparison of rexTEM to fibTEM results proved that peri-procedural hemostasis disorders in AVR result from procedure-related fibrinogen deficiencies, with no capacity for proper clot formation (lack of CFT in fibTEM) and significant reduction in clot firmness directly after the procedure (Figures 1, 2). After 24 h fibrinogen concentration and fibrin polymerization abilities were restored to basic values, which allowed for formation of a clot with proper firmness (Figure 2).
Figure 1
Changes in thromboelastometry rexTEM test related to AVR vs. TAVI. Comparison of median values of CT, CFT, A5-A30, MCF, LI, ML between cohorts

Figure 2
Changes in thromboelastometry fibTEM test related to AVR vs. TAVI. Comparison of median values of CT, CFT, A5-A30, MCF, LI, ML between cohorts

Patients subjected to TAVI had similar results in external pathway initiated hemostasis (rexTEM) with significantly prolonged clot formation (CFT; p = 0.0004), and reduced clot firmness at each stage of test running (A5-A25), including maximal clot firmness (MCF; p = 0.004) directly after the procedure. Time of plasma factor activation was not disturbed (CT; p = 0.55). Interestingly, 24 h after the procedure hemostasis had not been restored completely, proving still significantly prolonged clot formation (CFT; p = 0.05), and significantly reduced clot firmness (MCF; p = 0.019). On the other hand, fibrinogen concentration and fibrin polymerization were not substantially disrupted directly after TAVI. Comparison of rexTEM and fibTEM results suggested that the peri-procedural hemostasis disorders in TAVI cohort resulted mainly from platelet dysfunction (Figures 1, 2).
Comparison of the results between TAVI and AVR cohorts proved a similar range of external pathway hemostasis disorders directly after the procedures (Figure 1). However, 24 h after interventions, hemostasis in the TAVI cohort was still impaired in contrast to almost completely recovered hemostasis after AVR (Figure 1). Comparison of fibTEM and rexTEM results between cohorts showed that hemostasis alterations in the case of AVR result from fibrinogen dysfunction, and platelet dysfunction in the case of TAVI (Figures 1, 2). No enhanced clot lysis was observed early after the procedures.
Platelet reactivity
Results of ASPItest in AVR patients proved a significant decrease in platelet reactivity directly after the procedure (p < 0.0001), which returned to basic values at 24 h after it (p = NS). In the TAVI cohort platelet reactivity in ASPItest significantly decreased directly after the procedure (p < 0.0001), and remained significantly reduced also at 24 h after it, in comparison to initial values (p < 0.0001). Comparing results of both groups, TAVI patients had significantly higher sensitivity to aspirin before and after the procedure in comparison to AVR patients, in whom quick recovery to lower sensitivity to aspirin was noted (Figure 3 A).
Figure 3
Comparison of changes in platelet reactivity related to AVR vs. TAVI. A – COX-1 activity, B – P2Y12 receptor activity, C – PAR-1 receptor activity, D – vWF activity
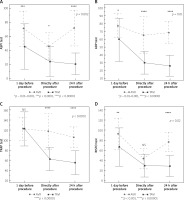
Results of ADPtest proved that only TAVI patients experienced a significant reduction in platelet reactivity directly after the procedure (p < 0.00001). Furthermore, this decrease persisted during the next 24 h (p < 0.00001, in comparison to initial values). In contrast, a negligible drop in platelet reactivity directly after and 24 h after the procedures characterized patients subjected to AVR (p = 0.1). Comparing platelet reactivity in both groups, initial sensitivity to P2Y12 inhibitors in TAVI patients was significantly greater (p < 0.01–0.001) and became ever more profound within 24 h after the procedure in comparison to AVR patients (Figure 3 B).
Furthermore, results of TRAPtest proved that although initial platelet reactivity in response to thrombin receptor activation was comparable, TAVI patients experienced a significant decline in platelet reactivity directly and at 24 h after the procedure (p < 0.00001), while in patients subjected to AVR no significant changes in platelet reactivity were noted (p > 0.1) (Figure 3 C).
In RISTOtest AVR patients had a significant decrease in vWF activity directly after the procedure (p < 0.00001), with significant recovery after 24 h (p < 0.00001). TAVI patients also experienced a significant decrease in vWF activity directly after the procedure (p < 0.0001). However, no recovery of vWF activity was noted at 24 h after TAVI (p = NS) (Figure 3 D).
Standard laboratory hemostasis parameters
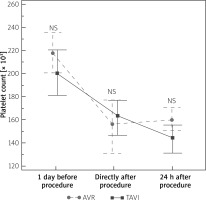
A significant reduction in platelet count after the procedures was observed in both cohorts. Comparing the results between groups, TAVI patients had a numerically lower platelet count before and 24 h after the procedure in comparison to patients subjected to AVR (Figure 4). Directly after the procedure platelet count was slightly higher in TAVI than in AVR cohort. However, none of these differences reached statistical significance. Hemoglobin concentration did not differ between groups. Activated partial thromboplastin time was significantly longer before the procedure in the TAVI cohort, while after the procedures the AVR cohort had higher APTT values (Table II).
Discussion
Due to ageing of populations and increasing prevalence of degenerative AS in highly developed countries, the number of AVR and TAVI procedures in Europe and North America is constantly increasing [7, 25].
Despite the high effectiveness, these procedures are invariably associated with increased risk of bleeding and thromboembolic events, with early bleeding as the most frequent [7–11]. In the search of causes of this phenomenon, currently attention is mainly focused on not fully evidence-based and arbitrarily recommended antithrombotic prophylaxis after these procedures [7, 26]. Peri-procedural hemostasis alterations associated with AVR and TAVI might be relevant for this phenomenon, likewise for the effectiveness and safety of peri-procedural antithrombotic prophylaxis. Yet, until now thorough analyses of hemostatic potential and platelet reactivity in the strict peri-procedural period of AVR and TAVI have never been undertaken.
It is known that patients with AS are affected by hemostasis alteration [1–6]. Initial hemostasis disorders related to AS are bidirectional, with proven high platelet activation and increased thrombin generation on one hand, and acquired deficiency of vWF or the platelet shedding phenomenon on the other [1–6, 27]. Additionally, the impact of advanced age on platelet function with increased platelet reactivity and depletion in plasma factor XIII were previously described [28–31]. Due to these alterations, AS patients are at increased risk of thromboembolic events and bleeding simultaneously [28, 31–33]. Given the fact that initially hemostasis disorders may overlap with disturbances related to the procedures themselves, the etiology of peri-procedural hemostasis disorders in AS patients seems to be multifactorial, complex, and not fully investigated.
Hemostasis disorders associated with AVR are partially explained by the use of extracorporeal circulation. The insufficient hemocompatibility of cardiopulmonary bypass (CPB) devices, which are negatively charged and not covered with endothelium, causes activation of plasma coagulation factors, platelets, complement components, endothelial cells and leucocytes [8, 16, 18, 27]. Subsequently, fibrinogen and vWF are adsorbed onto the surface of CPB, providing a nidus for platelet adhesion and aggregation. This results in constant activation and consumption of plasma coagulation system components expressed in hypofibrinogenemia, high platelet thrombogenicity, and a global inflammatory response [34, 35]. Non-physiological turbulent blood flow with high shear forces and areas of stasis in CPB potentiate these alterations [34, 35]. On top of this, dilutional coagulopathy and prolonged activation of coagulation factors caused by hypothermia have been proven [36]. Despite the substantial hemostasis disorders associated with AVR, quick restoration of vWF multimers and fibrinogen within 24 h after this procedure was reported [22]. Similarly, increased platelet reactivity with increased thrombogenicity within 3 months after AVR has been proven [3, 20, 21].
Data on hemostasis alterations associated with TAVI have considered only relevant thrombocytopenia, increased platelet activation, expressed in release of microparticles, and immediate restoration of vWF multimers after the procedure [12–15, 33, 37, 38].
The importance of our study is expressed in a few novel results.
We performed detailed analysis of platelet reactivity disturbances related to AVR and TAVI. Furthermore, we compared the two procedures, proving that the peri-procedural hemostatic profile of patients subjected to AVR differs in comparison to that of those subjected to TAVI.
In the case of platelet reactivity we found that patients subjected to AVR had only a transient increase in sensitivity to aspirin with quick recovery to baseline reactivity. Of note, this finding might not express the quick restoration of young, reactive platelets, but rather the result of constant activation related to CPB and heparin, as was suggested previously [30, 34, 35].
Furthermore, AVR patients presented significant, but only procedure-related depletion in vWF activity, with restoration within 24 h after AVR. This finding is contradictory to previous reports, which showed that reduced vWF activity concerns AS patients before AVR, with complete restoration within the next 24 h [34].
In the case of TAVI, we proved that the main cause of peri-procedural hemostasis disorders results from platelet dysfunction. We found significant, procedure-related impairment of platelet reactivity, which in contrast to AVR did not improve after 24 h.
Interestingly, platelet sensitivity to aspirin before and after TAVI was higher in comparison to AVR, although both cohorts received equal doses of aspirin before the procedures, and AVR patients received a double dose of aspirin after surgery. Similarly, initial sensitivity to P2Y12 inhibitors was significantly higher in the TAVI cohort and substantially increased after TAVI. This change was significantly stronger than after AVR, though none of the TAVI patients received clopidogrel before and within 24 h after the procedures. Furthermore, in contrast to the previous reports vWF activity after TAVI did not recover within 24 h, though no significant aortic regurgitation was found [13, 33].
It is difficult to compare our outcomes to studies which adopted different time points and parameters for hemostasis assessment after TAVI, especially when their results are divergent [12–15, 33, 37, 38].
Jung et al. observed that, 1 week after TAVI, serum concentration of platelet microparticles, a marker of platelet activation, was lower than before the procedure, which significantly increased afterwards [12]. Another, small sample size study investigated viscoelastic properties of clot formation early after TAVI [14]. So far, only one study has compared platelet function and global hemostasis in patients subjected to AVR and TAVI [15]. Similarly to our observations, the researchers observed a drop with quick restoration in platelet reactivity after AVR, and significantly impaired platelet function directly after TAVI [15], which did not recover to initial values until the last assessment. In contrast, fibrinogen function was only mildly impaired after AVR with rapid reconstitution to values which even exceeded the initial ones, and a significant procedure-related drop in platelet count occurred only in the AVR cohort [15].
We are aware that the results of platelet reactivity analyses may be altered by platelet count. Similarly as previous studies, we found that platelet count decreased significantly after both interventions [37–39]. Yet, the observed differences in platelet reactivity between AVR and TAVI cannot be simply explained by procedure-related thrombocytopenia, since both study cohorts had a similar platelet count at each time point of assessment.
A potential explanation of this phenomenon might be platelet receptor shedding related to the TAVI procedure [27, 35]. However, if so, the AVR procedure and shear forces of CPB should be more traumatic for platelets. Another cause of this finding might be diminished platelet regeneration after TAVI. It was proved that elderly people have reduced potential to regenerate blood components after bleeding, which was expressed in a reduced number of reticulated, more reactive platelets [40]. However, AVR is associated with greater blood loss than TAVI; therefore, impairment in platelet reactivity should be more profound in AVR patients.
Study limitations
Our study has an explorative and hypothesis-generating nature; therefore, the results should be perceived as preliminary. The explorative character determines the small sample size of the study, which together with limited diagnostic tools and time points of assessment do not allow us to draw too far-reaching conclusions. The small sample size was responsible for the high rate of noted bleeding. It also made it impossible to correlate discovered hemostasis disorders with hemorrhagic events. Furthermore, differences in procedures limit the value of comparative analysis of hemostasis disorders between the interventions. The two procedures also differ in terms of doses of UFH and expected level of ACT during the intervention. Thus, we did not analyze the results of inTEM tests, which express the impact of UFH and protamine on hemostasis. Durative hemostasis disorders may be responsible for early bleeding related to aortic valve interventions. Our study may become a basis for further thorough analyses, designed to verify this hypothesis.
Conclusions
Surgical and transcatheter and aortic valve procedures are associated with substantial and diverse peri-procedural hemostasis disorders. Surgical aortic valve replacement is characterized by hypofibrinogenemia and vWF depletion, which quickly recover within 24 h after the procedure. Transcatheter aortic valve implantation is characterized by early significant impairment of platelet function with increased sensitivity to antiplatelet agents, which is not recovered at 24 h after the procedure. Therefore early dual antiplatelet prophylaxis after TAVI requires careful consideration.