Introduction
Sequential improvements in image-guided radiotherapy (RT) for prostate cancer (PCa) have enhanced the precision of dose delivery to the prostate, and the modulation of dose around the adjacent ‘organs-at-risk’. Radiation-related rectal toxicity is a serious concern that may adversely affect health-related quality of life (HR-QoL), particularly given the increasing use of hypofractionation and focal dose-escalation. The radiotherapy dose delivered to the rectum can be reduced by spatial separation from the prostate through the implantation of a temporary gel in the avascular plane in between (i.e. perirectal spacer), with the goal of reducing patient-reported toxicity. However, despite its inherent logic, whether rectal spacing lowers the severity or alters the type of treatment-related adverse events (AEs) in patients treated with RT for clinically localized PCa remains unproven.
Based on a randomised clinical trial (RCT), the Food and Drug Administration approved the use of a perirectal hydrogel spacer (SpaceOARTM) device, intended to ‘reduce the radiation dose delivered to the anterior rectum [1]. However, there was no evidence off a statistically significant reduction in toxicity according to the predefined co-primary safety endpoint of the trial [2]. In the post-approval period, many case series and studies of self-reported AEs raised concerns about the safety in real-world setting. In particular, a recent Manufacturer and User Facility Device Experience (MAUDE) database analysis of the SpaceOAR device showed that, albeit rarely, perirectal spacers can lead to severe AEs such as abscesses, fistulas, or even death [3]. A meta-analysis suggested that perirectal spacers reduce late rectal toxicity, but the study was based primarily on data from retrospective studies [4]. Recently, 2 additional RCTs testing newly emerging BarrigelTM and ProSpaceTM devices presented their primary trial outcomes [5, 6].
In light of the increasing clinical use and the incomplete evidence, we conducted a systematic review and meta-analysis of prospective trials assessing the efficacy of perirectal spacers in reducing rectal toxicity from contemporary RT. We pooled the outcomes of RCTs and summarised studies that used perirectal spacers in a non-comparative manner to provide the readers with a comprehensive understanding of the evidence supporting their clinical use.
Material and methods
This systematic review and meta-analysis was prospectively registered in the PROSPERO database (CRD42024506380) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7]. The PRISMA 2020 checklists are included in Supplementary File 1 A. The study question and inclusion criteria were formulated using the Population, Intervention, Comparator, Outcome, and Study Design (PICOS) process. In brief, we aimed to assess the evidence from studies involving patients treated with RT for clinically localised PCa (population) using perirectal spacers (intervention), compared to no spacer (comparator), with regard to periprocedural safety and reduction in rectal AEs (outcomes), based on data from RCTs (study design). Evidence from non-randomised trials was retrieved separately and summarised qualitatively. The complete PICOS process can be found in Supplementary File 1 B.
We searched MEDLINE (via PubMed), Embase, and Scopus databases, and the Google Scholar search engine (top 200 hits retrieved) on 2024/03/14. No language or date restrictions were imposed. The database search strategy was updated on 2024/08/18 prior to peer review, using date filters to restrict the search to records published since March 2024. The detailed search strategy can be found in Supplementary File 1 C. For the quantitative synthesis, we retrieved full-text manuscripts and abstracts from major scientific conferences describing the results of RCTs investigating the clinical efficacy of rectal spacers in RT for localised PCa. In cases where multiple publications described a single trial, the most recent evidence reporting primary safety outcomes was retrieved. More than one publication was included if the reported evidence was complementary. Publications describing explorative and subset analyses or retrospective studies were excluded. In the case of conference abstracts, corresponding full-text reports were sought for retrieval. If no corresponding full text could be identified for a RCT report, the data was retrieved from the published abstract. Full-text manuscripts describing the outcomes of non-randomised prospective trials using perirectal spacers were retrieved separately. The retrieved records were de-duplicated and screened using the Rayyan.ai platform [8]. Abstract screening and the consecutive full-text screening were each performed independently by two authors. Conflicts were resolved through mediation with co-authors.
The data retrieved from the reports of RCTs included the first author’s name and year of publication, trial identifier, total number of patients and allocation ratio, basic inclusion and exclusion criteria, years of accrual, number and location of participating centres, RT details, device name and description, additional procedures necessary to implant the spacer, rates of procedural success, periprocedural toxicity, rates of early (0–3 months) and late rectal AEs defined according to the Common Terminology Criteria for Adverse Events and stratified by grade, additional bowel-related outcomes (such as patient-reported QoL), and dosimetric results. The risk of bias was assessed in RCTs using the Cochrane Risk of Bias 2 tool [9]. The main outcome was the incidence of grade 2 or worse early and late rectal AEs, presented using forest plots as pooled odds ratios (OR) with corresponding 95% confidence intervals (CI). To ease interpretation, AE reduction was additionally presented as a number needed to treat (NNT) to prevent one AE. The analyses were also performed for the incidence of grade 1 or worse rectal AEs. The rates of AEs, dosimetric outcomes, and basic characteristics of the included RCTs, and the characteristics of identified non-randomised studies were tabulated. Both data retrieval and risk of bias analysis were performed independently by two authors, and conflicts were resolved through mediation with co-authors.
The statistical analysis was performed using the R environment, version 4.4.1 [10], RStudio software build 764 [11], and the “meta” and “metafor” packages, along with basic statistical tools. The odds ratios for the occurrence of early and late grade ≥ 2 and grade ≥ 1 rectal AEs were pooled using the ‘metabin()’ function, applying the default generalised linear mixed model (GLMM) meta-analysis with a logistic regression model and maximum-likelihood estimator for Tau2. Random-effects model estimations were reported with respective 95% CI’s and p-value for the Z statistic, and presented on forest plots. The number needed to treat was calculated manually for the average OR estimations and corresponding 95% CI boundaries, using the formula presented in chapter 15.4.4.2 of the Cochrane Handbook for Systematic Reviews of Interventions, Version 6.4, 2023 [12]. The pooled rates of grade ≥ 1 or grade ≥ 2 rectal AEs in the control arms were used as the assumed comparator risk for NNT calculation. The assumed comparator risk was calculated using logit-transformed individual AE rates as input for the metaprop function, with the method set to GLMM, and then back-transformed for interpretation and further calculations. Rates of AEs in individual studies were compared using Fisher’s exact test. Heterogeneity was assessed using the I2 statistic (values > 50% considered significant) and Cochran’s Q test. Funnel plots were omitted due to the low number of included studies. All tests were two-sided. P-values < 0.05 were considered significant.
Results
Of the 848 individual screened study records, 4 reports describing the results of 3 multi-institutional RCTs (n = 645 pa- tients) (Table 1) [2, 5, 6, 13] and 22 reports of non-randomised prospective clinical trials (n = 1140 patients) (Suppl. File 1) [14–35] were identified, as shown in the PRISMA flow diagram (Suppl. File 1 D). Three additional records that initially appeared as relevant to the inclusion criteria were excluded due to the concerns regarding study design, as described in Supplementary File 1 E. Each of the 3 included RCTs allocated over 200 patients in a 2 : 1 ratio to receive standard-of-care RT with or without a perirectal spacer. The devices included SpaceOARTM absorbable polyethylene glycol hydrogel spacer [2], BarrigelTM absorbable hyaluronic acid gel spacer [5], and the ProSpaceTM biodegradable poly L-Lactide-cocaprolactone saline-filled balloon spacer, with the latter allowing for adaptable deployment [6]. None of the 645 included patients received elective pelvic lymph-node irradiation. Intensity-modulated RT or volumetric-modulated arc therapy was used to treat the whole gland, using either conventional fractionation or moderate hypofractionation. Two trials included patients primarily with intermediate-risk group characteristics [2, 5], while the third trial limited accrual to participants with localized T1-3 PCa without posterior extra-prostatic extension on magnetic resonance imaging (MRI) [6]. In each of these 3 trials, spacer implantation was performed in conjunction with antibiotic prophylaxis and anaesthesia, including general anaesthesia in 36.4% and 57.2% of the patients included in the studies investigating the SpaceOARTM and ProSpaceTM devices [2, 6].
Table 1
Summary of randomised controlled trials investigating the clinical effect of rectal spacer implantation before radiotherapy for prostate cancer
| Author, year | N, allocation | Number of centres and location | Device, short description, trial registration | Study duration | Basic inclusion criteria | RT details | Additional procedures (%) | Procedural success (%) | Periprocedural toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Mariados et al. [2] Pieczonka et al. [13] | 222, 2 : 1 | 20 centres in the USA | SpaceOARTM Absorbable polyethylene glycol hydrogel spacer; NCT01538628 | 2012–2013 | T1 or T2 Gleason score ≤ 7 PSA ≤ 20 ng/ml, and a ECOG 0 or 1 | 79.2 Gy in 1.8 Gy fractions, IG-IMRT, prostate only | Antibiotic prophylaxis (95) Anaesthesia: General (36.4) Sedation (31) Local (31.4) Other (10.5) | 99 | 10% of the patients experienced ‘mild procedural AEs’, such as perineal discomfort |
| Mariados et al. [5] | 201, 2 : 1 | 16 centres in the USA, UK, Portugal, Poland, Netherlands, and Israel | BarrigelTM Absorbable hyaluronic acid gel spacer; NCT04189913 | 2020–2021 | T1 or T2 Gleason score ≤ 7 PSA ≤ 20 ng/ml | 60 Gy in 3 Gy fractions, IG-IMRT/VMAT, prostate only | Antibiotic prophylaxis Local anaesthesia or conscious sedation | 100 | None reported |
| Song et al. [6] | 222 2 : 1 | 16 centres in the USA, Australia, and Spain | ProSpaceTM Biodegradable saline-filled balloon spacer composed of poly L-Lactide-cocaprolactone; NCT03400150 | 2018–2021 | T1, T2, or T3 without posterior extraprostatic extension on MRI | * 60 Gy in 3 Gy fractions (40.5%), 67.2-72 Gy in 2.4 Gy fractions (38.2%), 79.2-81 Gy in 1.8 Gy fractions (20.5%), Other fractionation (0.9%), IMRT/VMAT (IG unspecified), prostate only | 5-days of antibiotic prophylaxis Anaesthesia: General (57.2) Sedation (9.5) Local (33.3) | 99.5 | 6.9% of the patients experienced ‘mild nonrectal procedure-related AEs’, including transient hemospermia, dysuria, hematuria, and fatigue 4.2% of the patients had possibly-related AEs, including abdominal tenderness, acute urinary retention, and dysuria 2.1% of the patients had procedure-related constipation and pain in the rectal area One patient (0.7%) experienced procedure-related serious AE: rectal puncture, which led to Grade 2 rectal bleeding, and necessitated removal of the perirectal spacer |
Dosimetric outcomes, defined as reductions in the expected RT dose delivered to the rectum based on a comparison of RT delivery plans developed using computed tomography performed pre- and post-spacer implantation, were used as primary endpoints in each included RCT. Additionally, 2 studies included the comparison of grade ≥ 1 rectal AEs rates as a second primary endpoint [2, 6]. Given that these studies were only single-blinded, relying on asymptomatic or mildly symptomatic events requiring only observation appears as a limitation. Moreover, participants could unblind themselves by checking for the presence of the perirectal spacer. None of these studies included HR-QoL patient-reported outcomes as a primary endpoint. All 3 studies were assessed as having a moderate risk of bias due to the aforementioned limitations in allocation masking (Suppl. File 1 F).
Overall, the toxicity was low. Only three grade 3 events were recorded (0.5%): one in a patient with an implanted spacer and two in patients without spacers. Additionally, one patient died 2 weeks after spacer implantation due to a cerebrovascular event, which was assessed as unrelated to the procedure and was not recorded as an AE in the trial results [6]. In each trial, the early events were defined as those occurring within the first three months. Late events were defined as occurring either between 3 and 6 months [6], measured at 6 months [5], or between 3 and 15 months [2]. The pooled incidence of G ≥ 2 rectal AEs in the control group was 7.1% (95% CI: 3.6–13.4%) for early events, and 1% (95% CI: 0.2–3.7%) for late events (Suppl. File 2A, B). The pooled incidence of G ≥ 1 rectal AEs in the control group was 31.6% (95% CI: 21.4–43.8%) for early events, and 6.2% (95% CI: 3.6–10.4%) for late events (Suppl. File 2 C, D).
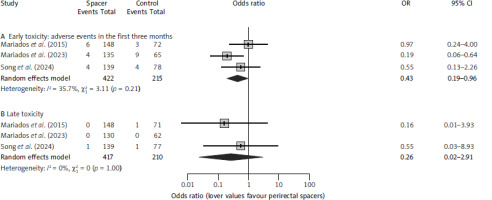
As presented in Table 2, no significant decreases in toxicity were observed for patients treated with perirectal spacers in individual studies, except for a reduction in acute G ≥ 1 (15.6% vs. 44.6%; p < 0.001) and G ≥ 2 rectal AEs (3% vs. 13.8%; p = 0.006) in the study that used exclusively moderate hypofractionation. However, by the 6 month assessment, none of the patients treated in this trial reported rectal G ≥ 2 AEs [5]. The pooled OR for developing an early rectal G ≥ 2 AE was 0.43 (95% CI: 0.19–0.96; p = 0.04) (Fig. 1 A). Assuming a 7.1% event probability in the control group, this corresponds to an NNT of 26 patients to avoid one event, ranging 18–378 patients (corresponding to the 95% CI of the OR estimation). The pooled OR for developing a late rectal G ≥ 2 AE was 0.26 (95% CI: 0.02–2.91; p = 0.27) (Fig. 1 B). Assuming a 1% event probability in the control group, this corresponds to a NNT of 135 patients to avoid one event, ranging from 104 patients to avoid one event to one additional harm per 54 patients.
Table 2
Clinical outcomes of rectal spacer implantation reported in randomised controlled trials
| Author, year | Acute rectal toxicity | Late rectal toxicity | Additional clinical outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–3 months | Spacer arm, n (%) | Control arm, n (%) | Fisher’s exact test* | 3–15 months | Spacer arm, n (%) | Control arm, n (%) | Fisher’s exact test* | ||
| Mariados et al. [2] Pieczonka et al. [13] | G0 | 108 (73) | 49 (68) | G0 | 145 (98) | 66 (93) | Proportion of patients with ≥ 10 points decline in EPIC bowel score (study vs. control group): At three months – 33.6% vs. 32.9% (p = N/A) At six months – 12.8% vs. 19.4% (p = N/A) At 12 months – 15% vs. 19.4% (p = N/A) At 15 months – 11.6% vs. 21.4% (p = 0.09) | ||
| G1 | 34 (23) | 20 (27.8) | 0.5 | G1 | 3 (2) | 4 (5.6) | 0.12 | ||
| G2 | 6 (4.1) | 2 (2.8) | > 0.9 | G2 | 0 (0) | 0 (0) | 0.3 | ||
| G3 | 0 (0) | 1 (1.4) | N/A | G3 | 0 (0) | 1 (1.4) | N/A | ||
| Mariados et al. [5] | G0 | 114 (84.4) | 36 (55.4) | G0 | 129 (99.2) | 57 (91.9) | Proportion of patients with ≥ 5 points decline in EPIC-26 bowel score (study vs. control group): At three months – 26.5% vs. 37.7% (p = 0.13) At six months – 22.7% vs. 26.2% (p = N/A) | ||
| G1 | 17 (12.6) | 20 (30.8) | < 0.001 | G1 | 1 (0.8) | 5 (8.1) | 0.014 | ||
| G2 | 3 (2.2) | 9 (13.8) | 0.006 | G2 | 0 (0%) | 0 (0) | > 0.9 | ||
| G3 | 1 (0.7) | 0 (0) | N/A | G3 | 0 (0) | 0 (0) | N/A | ||
| Song et al. [6] | G0 | 119 (85.6) | 62 (79.5) | G0 | 138 (99.3) | 74 (96.1) | Proportions of patients with declines in EPIC-26 domains analysed using mixed model analyses. No statistically significant differences were identified compared to baseline at any time point for any domain. | ||
| G1 | 16 (11.5) | 12 (15.4) | 0.26 | G1 | 0 (0) | 2 (2.6) | 0.13 | ||
| G2 | 4 (2.9) | 4 (5.1) | 0.5 | G2 | 1 (0.7) | 1 (1.3) | > 0.9 | ||
| G3 | 0 (0) | 0 (0) | N/A | G3 | 0 (0) | 0 (0) | N/A | ||
Fig. 1
Meta-analysis of reductions in the rates of early (A) and late (B) grade 2 or higher rectal toxicities
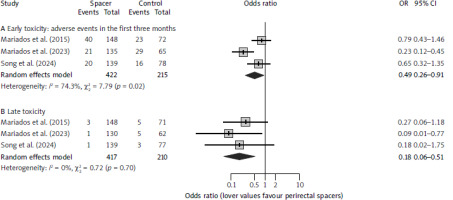
The meta-analysis for any grade rectal AEs, including grade 1 events, showed a significant decrease in patients with perirectal spacers. The odds ratios for developing a G ≥ 1 AE was 0.49 (95% CI: 0.26–0.91; p = 0.024) (Fig. 2 A) for early, and 0.18 (95% CI: 0.06–0.51; p = 0.001) (Fig. 2 B) for late events. Assuming a pooled incidence of 31.6% for any early and 6.2% for any late AE in the control groups, the corresponding NNTs would be 8 (ranging 5–50) to avoid one early G ≥ 1 AE, and 20 (ranging 17–34) to avoid one late G ≥ 1 AE.
Fig. 2
Meta-analysis of reductions in the rates of early (A) and late (B) grade 1 or higher rectal toxicities
Each included RCT evaluated how the implantation of the perirectal spacer affected the RT dose delivered to the rectum. These outcomes were assessed through computer simulations in the RT treatment-planning system, using computed tomography scans performed pre- and post-spacer implantation as an input. Based on those, two RT plans were developed and compared. The results were presented as reductions in predefined dose-volume parameters ranging V38–V80. The relative reductions in each of these parameters exceeded 50%, as shown in Supplementary File 1 G.
As shown in Table 1, the authors evaluated HR-QoL outcomes using the expanded prostate cancer index composite (EPIC) questionnaire. Despite numerical differences favouring perirectal spacers, there was insufficient evidence in the prespecified analyses to confirm a statistically significant improvement in the EPIC questionnaire’s’ bowel domain compared to the control groups.
There were no concerns regarding study heterogeneity, except for the analysis of early G ≥ 1 rectal AE reduction (I2 = 74% and p-value for Cochran’s Q = 0.02) (Fig. 2 A). This could be associated with the fact that in this study, the authors used exclusively moderate hypofractionation (60 Gy delivered in 3 Gy fractions), which has been previously shown to moderately increase rates of acute AEs [36]. Sensitivity and moderator analysis were omitted due to the low number of studies included in the meta-analysis.
Finally, there were 22 reports of non-randomised prospective clinical trials using perirectal spacers (n = 1140), as summarised in Supplementary File 1 H [14–35]. The majority of the patients included in these trials were treated in Europe (n = 392), followed by the USA (n = 347), Australia (n = 207), and Asia (n = 194). Most studies used the SpaceOAR system (n = 790) [14–16, 18–22, 25, 26, 28, 32, 35]; however, several other devices were used, such as the ProSpaceTM (n = 40) [23, 34], NASHA Spacer GelTM (n = 36) [17], Macrolane VRF30TM (n = 101) [27, 29], BioProtectTM (n = 33) [30], TraceITTM (n = 30) [31], and BarrigelTM (n = 31) [32]. The device was unspecified in 2 reports (n = 79) [24, 33]. The rates of successful implantations ranged 90.9–100% in individual studies. In the majority of these trials, perirectal spacers were used in the setting of conventional fractionation (n = 653) [14–16, 18–21, 23, 28, 29, 34], followed by ultra- hypofractionated RT (n = 329) [22, 24, 26, 30, 32, 33, 35], moderate hypofractionation (n = 36) [17], and high-dose rate brachytherapy (n = 33) [27]. In 2 trials, patients treated with different RT methods were included (n = 89) [25, 31]. In most cases, the authors reported only low-grade events, or no toxicity related to the perirectal spacer implantation. However, there were cases of clinically-relevant device-related AEs, such as rectal mucosal necrosis [16], G3 haematoma requiring surgical intervention [17], rectal wall injection [18], G3 rectal discomfort and pain [28], G3 rectal pain and ulcer [28], rectal bleeding [29, 32], or fever [29].
Discussion
In this manuscript, we summarise the available evidence from prospective trials for the use of perirectal spacers in RT for localised PCa. We found that the rates of moderate rectal AEs were low overall, even in control groups, particularly with regard to late events. Serious or worse rectal AEs were especially rare. Perirectal spacers significantly reduced the estimated dose delivered to the rectum in silico simulations, and were associated with a significant reduction in low-grade acute and late rectal AEs, which accounted for the majority of events recorded in these analyses. However, while there was a moderate decrease in the rates of acute rectal G ≥ 2 AEs in patients with perirectal spacers, the difference was not statistically significant for late events, and it did not translate into significant improvements in tested HR-QoL metrics. The success rates of perirectal spacer implantations were consistently high, but the procedure was associated with the need for antibiotic prophylaxis and anaesthesia. While most trials did not report significant spacer-related AEs, there were cases of clinically relevant events in the single-arm trials, and even a minor risk of additional treatment toxicity should be carefully weighed against the modest clinical benefit suggested by the available RCTs. It remains an open question whether the additional risks and costs justify the high number of patients needing to receive a perirectal spacer to avoid one G2 or worse rectal AE.
A recent analysis of the MAUDE database concerning SpaceOAR indicated that during the 2015–2022 period, in which over 200,000 devices were sold, there were 123 surgical and life-threatening (G ≥ 3) events reported, 5 of which resulted in death [3]. As these studies are potentially limited by reporting bias, the true prevalence of spacer-related toxicity remains unclear; however, the increasing number of such analyses indicates interest in pursuing the subject of emerging reports of harm associated with perirectal spacers [37, 38]. Despite the low absolute incidence, they represent a non-negligible safety signal for a device that does not aim to improve oncological outcomes. Some prospective studies also reported clinically relevant toxicities related to the spacer implantation, as described earlier in the manuscript [16–18, 28, 29, 32]. Most importantly, the implantation of a perirectal spacer could itself be regarded as a grade 3 AE, easily avoidable by omitting the procedure [39]. In the absence of a clear clinical benefit even at the threshold of avoiding G2 or worse AEs, let alone G3 or worse AEs, greater scrutiny of associated risks and benefits is necessary.
Moderate hypofractionation protocols have been evaluated for non-inferiority, unlike some other RT modifications designed to improve oncological outcomes [40]. For instance, higher rates of biochemical control can be achieved through dose escalation with brachytherapy-boost, at the cost of increased morbidity [41], or through focal integrated external-beam boost to the intraprostatic tumour as demonstrated in the FLAME trial [42]. In the latter, however, the authors did not report significant increase in toxicity, despite neither of the trials using perirectal spacers. Perirectal spacers could represent a useful tool to improve the risk to benefit ratio for moderately hypofractionated regimens. Nonetheless, modern RT for clinically localised PCa can be very safe even using sophisticated ultra-hypofractionated regimens, as shown in the PACE-B trial [43]. The dose delivered to organs at risk can be minimised by using more precise methods, and by reducing the treatment margins added to create the planning target volumes (PTV). The included RCTs generally allowed for wide choice of margins between 5–10 mm margins, depending on institutional preferences. Using margins closer to the upper limit could result in noticeably larger PTVs than those described, for example, in the MOMENTUM study, which evaluated MRI-guided adaptive RT achieving minimal gastro-intestinal toxicity [44], or those in the ultra-hypofractionated arm of the PACE-B study [43]. Similarly, reducing PTV, improving technological precision, and implementing daily image guidance allow us to achieve more stringent dose constraints for the organs at risk while maintaining sufficient PTV coverage [45]. Therefore, it is possible that the moderate acute toxicity reported in the control group of the study using spacers in the moderate-hypofractionation setting, which presented the highest rates of grade 2 or worse AEs, could be reduced by simply lowering the dose constraint limits [46]. On the other hand, it could be hypothesised that perirectal spacers would be more effective in patients with higher baseline risk of severe toxicity [47], such as those with inflammatory bowel disease [48], or patients undergoing local re-irradiation [49]. These patients could be a target for future research in rectal spacing; however, as presented in Supplementary File 1 H, we did not identify any published evidence from prospective trials investigating perirectal spacers in these setting.
There are several limitations to this meta-analysis. First, 2 of the 3 included RCTs primarily evaluated conventionally fractionated RT for clinically localised PCa, which, although safe, is gradually being replaced by hypofractionated RT regimens. Second, many of the patients included in these trials would now be candidates for active surveillance, which could further reduce the rates of AEs. Third, none of the patients in the RCTs were treated with more aggressive regimens, such as elective pelvic irradiation, target lesion boosting, or combined dose-escalation methods (e.g. brachytherapy boost), all of which could be associated with an increased risk of clinically significant AEs. Fourth, the reported follow-up periods were relatively short in comparison to the life expectancy of patients with localised PCa, and it is possible that perirectal spacers could significantly impact the rates of late-onset AEs. Finally, we did not perform cost-effectiveness analyses, which would require estimating the costs of devices, personnel, and peri-procedural care, all of which can vary significantly by region. However, considering that there was insufficient evidence to show a significant reduction in toxicity except for the rates of mild AEs and acute moderate AEs, it is possible that allocating spacer-related funds to providing more modern RT techniques (e.g., MRI-guided RT) could result in equal or superior clinical outcomes.
Conclusions
The available evidence indicates that, despite a clear dosimetric benefit, perirectal spacers result in only a small decrease in acute rectal toxicity of moderate or worse grade. There was insufficient evidence to show a statistically significant decrease in the rates of late moderate or worse AEs, or any severe AEs, both of which are uncommon in modern RT for localised PCa. It remains unclear whether perirectal spacers lead to a significant improvement in HR-QoL outcomes. These findings suggest a need for clearer information regarding the risks, benefits, and healthcare costs of perirectal spacers. Future research should focus on identifying patients at high risk for rectal toxicity, such as those receiving very high cumulative doses of RT, to assess the potential role of perirectal spacers in mitigating AEs.







