Introduction
The most frequent causes of pleural effusions are infections in childhood. Malignant thoracic neoplasms, particularly in infancy, are rare in the differential diagnosis of pleural effusion. Although rhabdomyosarcoma (RMS) is an uncommon childhood malignancy, it is the most frequently encountered soft tissue sarcoma found in infants and children. RMSs can exist anywhere in the human body, including head and neck, extremities, genitourinary tract, and trunk as the most common primary sites [1–3]. The alveolar type of RMS is one of the subtypes of RMS that has a poor prognosis. It occurs at a rate of 20% in all of RMS cases. The tumour pathophysiology is extremely close to the lungs; therefore, the tumour is also known as alveolar rhabdomyosarcoma (ARMS). Congenital ARMS, defined as disease present at birth, is a rare subtype of RMS [4, 5]. We present a case of congenital ARMS, in which the patient rapidly improved just after the first chemotherapy cycle.
Case report
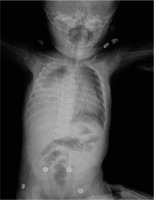
A four-month-old girl was admitted to the emergency department (ED) with complaints of respiratory distress without a runny nose and cough. In her history, she had a rash while having a bath one day earlier. In her physical examination, there was tachypnoea (40 breaths per minute) and a slight tachycardia (120 beats per minute) with no fever. Respiratory sounds in the right lung were diminished. Pleural effusion on the right hemithorax and pneumothorax on the left side was detected in the patient’s chest X-ray (Fig. 1).Complete blood count and routine biochemical tests were within normal limits. The levels of C-reactive protein (CRP) and procalcitonin were normal, with no signs of infection.
Fig. 1
Chest X-ray showing pleural effusion on the right hemithorax and pneumothorax on the left side.
A full panel viral swab culture was taken from the patient in order to rule out viral infection. The result of the swab culture was positive in terms of rhinovirus. A thorax tube was inserted for the pneumothorax in the left hemithorax, and thoracentesis was performed on the right side. Puncture fluid with 100 cc volume was transudate in character. The patient was transferred to the paediatric intensive care unit (PICU) for differential diagnosis and intensive treatment. On the second day of PICU admission, the effusion redeveloped in the right hemithorax of the patient. Therefore, a thorax tube was inserted into the right hemithorax. The tube on the left side was removed on the third day of PICU admission. Despite draining approximately 100 cc of fluid from the right hemithorax, effusion persisted for the following two days, and video-assisted thoracoscopic surgery (VATS) was performed to determine the aetiology of the persistent effusion. Pleural thickening with multiple nodules on the diaphragm was detected during VATS operation, and a Tru-Cut biopsy was obtained from the nodules. PAX3/FOXO1 fusion gene analysis confirmed the alveolar subtype and the poor prognosis of the patient. For staging purposes, the patient underwent thoracic and abdominal computed tomography (CT). A 55 × 32 × 35 mm heterogeneous semisolid mass, which compresses the liver in the subdiaphragmatic area, was detected (Fig. 2). There was also nodular thickening with 12 mm size on the right hemithorax pleural face with large pathological lymphadenopathies in the mediastinum (Fig. 3) and around the portal vein of the abdomen. A bony metastasis was detected at the L5 vertebra. Bone marrow aspiration was within normal limits. The pathology resulted in alveolar type RMS consistent with myogenin and desmin positivity (Fig. 4). Thus, the patient was classified as group IV according to the Intergroup Rhabdomyosarcoma Study Group (IRSG) classification, and as stage IV according to TNM pretreatment staging classification [6]. A vincristine, actinomycin-D, and cyclophosphamide (VAC) chemotherapy regimen was started. Because the patient had a plevral effusion during the VATS procedure, a third thorax tube had to be inserted in the right hemithorax. The patient responded to the chemotherapy, and during 10 days of PICU admission the effusion subsided gradually, and all the tubes were taken out. The patient was discharged on the 12th day of PICU admission, and she is still under chemotherapy and followed by the paediatric haematology and oncology division. After four cycles of chemotherapy, the majority of the primary tumour and the metastasis of the pleura, abdominal LAP, and the bony metastasis at the vertebra regressed. At the tumour board, since a very morbid surgery of right hemi pleurectomy was recommended as the local therapy, we decided to continue with chemotherapy only. Radiotherapy (RT) was not planned due to the very young age of the patient.
Fig. 2
Contrast-enhanced thorax CT showing a tumour 55 mm × 32 mm × 35 mm heterogeneous semisolid mass compressing the liver in the subdiaphragmatic area.
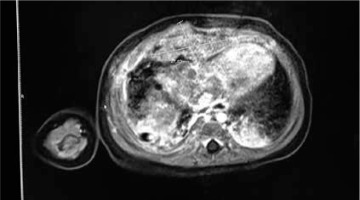
Fig. 3
Contrast-enhanced thorax CT showing 12 mm diameter nodular- enhanced solid lesions on the right hemithorax inferior pleural face.
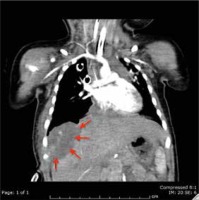
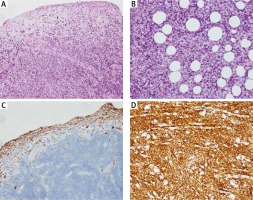
Fig. 4
A) Tumour infiltration consisting of fibroblasts in myxoid stroma in the sub-mesothelial area and small round cells lined with a flattened mesothelium on the surface (×100). B) Tumoural cells with clear cytoplasm infiltrating into adipose tissue (HE×200). C) Non-negative staining of positive tumoural cells in pleural plates on the surface with Oscar Keratin dye (Oscar Keratin 100×). D) MYOD1 positivity in tumoral cells (MYOD ×200)
Discussion
RMS usually occurs in the first two decades of life. In 5–10% of cases, it is diagnosed in children aged less than one year, and congenital RMS occurs in < 1% of all patients with RMS [7, 8]. Only a few cases (less than 20) have been reported in the literature regarding the alveolar subtype of congenital RMS. Various reports indicate that, when the diagnosis occurs in the neonatal period, the prognosis is worse than in older children [9–11]. The most frequent primary site for ARMS is the extremities, whereas congenital ARMS is a highly malignant tumour, often occurring as a disseminated disease [12, 13]. Likewise, in the literature, our case has poor prognostic characteristics: the alveolar type, large size (> 5 cm) and unfavourable localisation of the primary tumour, and presence of metastasis. Despite the fact that VAC is the gold standard for combination chemotherapy in the treatment of RMS, there are no specific chemotherapy guidelines regarding the treatment for neonates and infants with sarcoma. Infants with RMS are usually treated according to the same protocols used for older children: mainly alkylating agents, vincristine, and actinomycin-D, with or without anthracyclines [12–16]. However, they require age- and weight-adapted doses, given the physiological immaturity of various organs. Pleural effusion regressed, and the thoracic tubes could be taken out just after the first VAC regimen. The primary tumour and the metastasis responded very well after four cycles of chemotherapy with no significant toxicities. Due to possible sequelae, local control, determined by surgery and radiotherapy, also has unique challenges in very young children. In the literature, only three out of 16 patients with congenital ARMS received RT associated with conservative surgery. No conclusion can be drawn concerning the outcome of these small numbers of cases and the lack of follow-up [13]. The Italian Cooperative Group reported a higher local recurrence rate in infants with RMS, who did not receive appropriate local treatment [16]. In our patient, because radiotherapy was not recommended, we decided to continue chemotherapy to 40 weeks. Molecular gene profiling is even more critical in congenital forms of the tumours; despite the fact that the presence of the PAX3/FOXO1 fusion gene shows poor prognosis, other more in-depth biological studies are crucial for congenital RMS patients.
Conclusions
Our case is a rare alveolar subtype of congenital RMS occurring in the pleural localisation. This is a highly malignant tumour with an abysmal prognosis with almost no long-term survivors. Physicians should be aware of such an unusual presentation of congenital RMS, the diagnosis of which required recurrent pleural tube drainage and internalisation to PICU due to persistent pleural effusion. Although no specific chemotherapy guidelines regarding the treatment for infants with sarcoma exist, good chemotherapy responses are achieved with the regimens used for older children. Molecular biology studies are mandatory for tumour characterisation and the discovery of new therapeutic targets.








