Introduction
A radical transformation in diabetes therapy began over two decades ago with the development of the first continuous glucose monitoring systems. These innovations have fundamentally changed how patients and healthcare providers approach diabetes management and glycemic control. Continuous glucose monitoring (CGM) systems offer real-time insights into blood glucose fluctuations by continuously measuring glucose concentrations in the interstitial fluid [1]. This technology enables patients to monitor their glucose levels more effectively, supports safer self-management, and aids in optimizing diabetes treatment. The use of CGM systems has demonstrated a reduction in the risk of severe hypoglycemia and long-term complications, such as vascular disorders [2, 3].
Globally, and in Poland, the CGM systems market is growing. Access to CGM systems is expanding due to increased popularity, technological advances, and improved affordability. Over the past two years, Poland has experienced dynamic changes in CGM reimbursement policies, significantly enhancing access for individuals treated with intensive insulin therapy.
However, this growing diversity of available systems makes the selection process challenging for patients and medical staff alike. The outcome of close collaboration between patients and their therapeutic teams is crucial to identify the most appropriate CGM system, ensuring that patient preferences and individual needs are respected. CGM systems are recommended to aid in blood glucose control and improve the quality of life for patients with diabetes. Choosing the right system depends on factors such as age, fear of punctures, physical activity, aesthetics, and financial possibilities. Successful selection relies heavily on dialogue between patients, their guardians, and healthcare teams, particularly as modern CGM systems offer remote monitoring and integration with mobile applications and insulin pumps. Addressing patient concerns during the selection process is essential for achieving long-term treatment success.
Aim of the study
To review existing CGM systems and identify factors that influence personalization of CGM system selection for a patient with diabetes based on the functions and features of these systems.
Material and methods
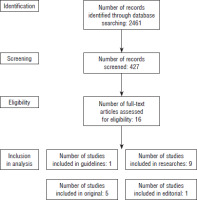
This analysis is based on sources from medical databases (PubMed), commercial studies (Dexcom, Medtronic, Ascensia Diabetes Care, Abbott, Genexo), and patient opinions gathered from internet forums (mojacukrzyca.org, mojacukrzyca.pl, Dexcom Polska Users, and Minimed 780G/640G Klub Polska) between 2018 and 2024 (Fig. 1). The search strategy included combinations of the following key words: “CGM,” “FGM,” “rt-CGM,” “is-CGM,” and “smart MDI.” Scientific publications were selected based on relevance to CGM use, device choice factors, and patient experience reports. Papers lacking the factors mentioned above were rejected. A total of 16 peer-reviewed publications were included, complemented by information from official device documentation and patient feedback.
Discussion
According to current recommendations from Diabetes Poland (PTD), the American Diabetes Association (ADA), and the International Society for Pediatric and Adolescent Diabetes (ISPAD), CGM systems are strongly recommended as tools for monitoring blood glucose safely and effectively [4]. The therapeutic team’s detailed analysis supports achieving glycemic goals safely while optimizing treatment strategies.
Successful diabetes management using CGM systems relies on a holistic approach that includes effective communication between patients, their guardians, and the therapeutic team. Education and awareness regarding CGM system functionality are essential for both patients and healthcare providers to achieve optimal treatment outcomes [5].
While each CGM system provides real-time glucose data, understanding the nuances between systems is vital for personalized diabetes management.
A patient-centered perspective
Patient age significantly influences CGM system selection. Not all systems are approved for pediatric use under the age of 18 according to manufacturers’ guidelines. Additionally, younger children often require daily assistance from legal guardians to manage the system effectively. Studies have reported that children with type 1 diabetes often have suboptimal glycemic control, partly due to parental fear of hypo- and hyperglycemia. The introduction of CGM systems has improved glycemic outcomes and enhanced the confidence of both patients and caregivers in managing hypoglycemia [8].
Hypoglycemia alarms provide substantial comfort and reduce anxiety for patients and guardians alike [9]. Moreover, the possibility of data sharing with multiple caregivers enhances security, ensuring continuous access to glycemic data and minimizing the fear of acute complications (Table I).
Table I
CGM systems available on the Polish market and their parameters
| Name of the system | Dexcom One+ | Dexcom G7 | Dexcom G6 | Guardian Link 3 | Guardian Link 4 | Guardian Connect | Simplera | Eversense E3 1 | FreeStyle Libre 2 | FreeStyle Libre 2 Plus | AnytimeCT3 | Touchcare S9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brand | Dexcom | Dexcom | Dexcom | Medtronic | Medtronic | Medtronic | Medtronic | Eversense | Abbott | Abbott | Genexo | Medtrum |
| MARD (%) | 8.7 | 8.2 | 9 | 8.7 | 8.7 | 8.7 | 10.2 | 8.5 | 7.9 | 8.2 | 9.4 | 9.7 |
| Sensor working time (days) | 10 | 10 | 10 | 7 | 7 | 7 | 7 | 1802 | 14 | 15 | 14 | 14 |
| Possibility of calibration | No | No | No | Yes | No | No | No | Yes | No | No | Yes | No |
| Possibility of calibration | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Effect of paracetamol on measurement accuracy [6] | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Sensor start-up period [h] | 0.5 | 0.5 | 2 | 2 | 1 | 2 | 2 | 24 | 1 | 1 | 3.17 | 0.5 |
| Transmitter (charging) | Built into the system | Built into the system | Once every 3 months, charging not possible | Possible charging, replaced once a year | Possible charging, replaced once a year | Possible charging, replaced once a year | Built into the system | Once a year, charging not possible | No transmitter | No transmitter | Once every 112 days, charging not possible | Once every 365 days, charging not possible |
| Sensor size [mm] [5] | 24.1 27.4 4.7 | 24 27.3 4.6 | 45.7 30.5 15.2 | 38 52 | 38 52 | 38 52 | 28.65 28.65 | 37.6 48 8.8 | 2.9 21 | 2.9 21 | 33.1 19.35 8.3 | 18.5 17.8 4 |
| Integration with insulin pump | No | No | Yes | Yes | Yes (only version for 780G system) | No | Yes (only version Sync) | No | No | Yes | No | No |
| Compatibility with smartphone | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Compatibility with smartwatch | Yes | Yes | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| Integration with electronic insulin pen | No | No | No | No | No | No | Yes | No | Yes | No | No | No |
| Compatibility with a dedicated receiver | Yes | Yes | Yes | No | No | No | No | No | Yes | No | No | No |
| Registration age | 2 | 2 | 2 | 7 | 7 | 7 | 7 | 18 | 0 | 2 | 18 | 2 |
| Maximum number of therapy partners | 10 | 10 | 10 | 5 | 5 | 5 | 5 | 5 | 20 | 20 | 10 | – |
| Possibility of use during pregnancy | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes |
Fear of puncture is another key factor affecting system selection. For patients sensitive to frequent pricking, systems that do not require calibration are preferable. However, systems with optional calibration may provide greater accuracy by allowing the user to manually adjust glucose readings twice daily. Sensor insertion involves skin puncture; thus, systems with different sensor replacement intervals – 7, 10, 14, or 180 days – offer flexible options based on individual needs (Table I).
Physical activity is a crucial component of diabetes management. CGM systems support the prevention of exercise-induced hypoglycemia and nocturnal hypoglycemia after activity. The choice of insertion site must accommodate body movement and exercise type. Athletes, particularly professional swimmers, may benefit from long-term sensors that minimize disruption during training, such as 180-day systems.
Aesthetic concerns can impact patient acceptance of CGM systems, particularly among adolescents and young adults. The visibility of devices, potential influence on body image, and intimacy concerns with partners can all affect device adoption [7]. Placement in discrete locations or the choice of smaller, less obtrusive devices can alleviate these issues.
Financial constraints significantly impact CGM adoption. Systems vary widely in price, and maintenance costs include sensors, receivers, smartphones, or smartwatches. Some systems come with dedicated receivers, offering alternatives to expensive smartphones. Financial discussions should be part of the CGM system selection process to ensure sustainable use.
Communication between the therapeutic team and the patient
The use of the CGM system facilitates the patient’s cooperation with the diabetes care system, even remotely. Modern diabetes care requires the selection of a CGM with the function of easy, clear reading and transferring data from at least the last 90 days to the therapeutic team. Elements improving communication may also include: simplified vocabulary, an individual approach, and visual materials to illustrate the available systems and their diversity better.
Compatibility
The use of therapy requires cooperation with the application and/or insulin pump. This connection allows the patient to constantly control the data and quickly respond to changes in blood glucose values. The app allows the patient to view the current glycemia status and observe the trend and chart, and is responsible for notifications. It allows sharing of data with care partners and constant monitoring of data from the patient’s system on their device [10]. The number of care partners varies according to the system type. Some CGM systems are compatible with a smartwatch with easy viewing of blood glucose values without having to remove the smartphone or pump.
Integrated CGM systems with insulin delivery devices
An important feature of some CGM systems is the integration with the insulin pump. Integrating both adequate devices opened the way to closed-loop therapy [11, 12], including systems such as low-glucose suspend, predictive low-glucose suspend [13], and the latest innovation, AID [14] systems. In all of them, the CGM system plays a key role. Thanks to this, the use of AID is associated with better therapeutic effects while enhancing quality of life [15]. In addition, integrated systems, particularly automatic insulin delivery (AID), have significantly improved patients’ glycemic control.
For people with diabetes treated with multiple daily injections (MDI), choosing a CGM integrated with a pen will be the most beneficial. Two of the CGM systems available in Poland are compatible with the smart pen (Table I). The InPen system, for example, supports insulin application records, doses and time of injection, including active insulin tracking; dose calculators that provide individualized dosing recommendations; and alerts for missed doses; also, in the case of changes in glycemic values, it suggests ing action to correct the blood glucose level [16].
Conclusions
Continuous glucose monitoring systems are strongly recommended by diabetes organizations as tools for improving blood glucose control and enhancing patient quality of life. Personalized CGM selection depends on multiple patient-specific factors, including age, fear of puncture, physical activity, aesthetic concerns, and financial capacity. Close cooperation between patients, guardians, and the therapeutic team is essential to achieve optimal system selection. Addressing patient concerns regarding hypoglycemia, device visibility, and costs through comprehensive consultations can significantly enhance adherence and therapeutic outcomes.

 POLSKI
POLSKI