Introduction
Injuries of the descending aorta are treated commonly by thoracic endovascular aortic repair (TEVAR) [1, 2]. Arch injuries are qualified for an open procedure called ‘frozen elephant trunk’ (FET) with cardiopulmonary bypass, hypothermia and temporary circulatory arrest [1]. This operation requires complete heparinization and hypothermia (about 28°C) which cause additional injury. Patients after injuries of parenchymal organs with active bleeding and temporary tamponade (damage control) cannot be treated with FET. Instead the physician-modified endograft is an available option for arch repair in some centers. Moreover, endovascular technology will be even more popular when off-the-shelf devices for complex endovascular arch repair become available on the market.
Case report
A 27-year-old man after a polytrauma (motorcycle accident) was admitted to the hospital. Splenectomy and liver packing (damage control by 7 surgical scarves) due to liver damage was performed. Moreover, the patient’s kidney was torn off, and he suffered from multiple tears of the intestinal mesentery and rupture of bile ducts. After transfusion of concentrated red blood cells and plasma 1 : 1 the patient was stabilized. Additionally, he broke his left femur and left forearm (equipped with a rail) with significant damage of the muscle mass, resulting in myoglobinuria. The aortic arch was ruptured just behind the brachiocephalic trunk (border of zones 1 and 2) with complete separation of the aorta, subdural hematoma and left pleural hematoma (Figure 1 A). The injury location required aortic arch replacement by an open FET procedure. The patient would not survive cardiopulmonary bypass, hypothermia and full-heparinization surgery lasting several hours due to coexisting injuries. Therefore, he was qualified for fenestrated thoracic endovascular aortic repair using a physician-modified endograft (FTEVAR-PMEG) as a damage control procedure.
Figure 1
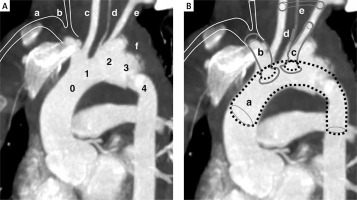
A – Computed angio-tomography of the aortic arch. 0 – Zone: bovine arch common orifice of the brachiocephalic trunk (size 10 mm and 48 mm length do its bifurcation) and left common carotid artery. 1 – Zone: due to the common departure of the trunk and the left carotid artery, zone 1 is located inside zone 0. 2 – Zone: left subclavian artery (size 7 mm and length 30 mm to the first collateral) comes from pseudoaneurysm. 3 – Zone: Short segment (about 2 cm) of the thoracic aorta just behind the left subclavian artery (it is the common landing zone for TEVAR; in this case the zone also includes pseudoaneurysm). 4 – Zone: this segment is partially involved in rupture. a – marked position of the right subclavian artery (not visible on the reconstruction due to angular settings). b – marked position of the right common carotid artery (not visible on reconstruction due to angular settings). c – left common carotid artery. d – left vertebral artery (3 mm) departing from the aortic arch (in this case from the pseudoaneurysm). e – left subclavian artery. f – hematoma around the aorta. B – FTEVAR-PMEG plan. a – Medtronic stent graft (VAMF2622C150TE). b – VBX stent graft (8 × 80 mm). c – LifeStream stent graft (8 × 45 mm). d – Amplatzer (6 mm) in the left vertebral artery. e – Carotid-subclavian bypass made of (8 mm PTFE ringed) prosthesis
The Valiant VAMF2622C150TE stent graft (Medtronic, Minneapolis, MN, USA) was modified by cutting fenestrations in it for the brachiocephalic trunk with a diameter of 9 mm and for the left subclavian artery with a diameter of 6 mm. The distance between the fenestrations was set at 1.2 cm. Both fenestrations were made in the maximal dorsal position of the stent graft. Additionally, occlusion of the left vertebral artery was done with the 6 mm (there was not smaller one – 3–4 mm) Amplatzer (Abbott, Plymouth, USA) before stent graft implantation to stop reverse flow to the aneurysm. The procedure was performed under general anesthesia. Femoral arteries were accessed percutaneously with the ProGlide system (Abbott, Plymouth, USA). 50 mg of heparin was administered and the activated clotting time (ACT) was maintained within 100 s. The left subclavian and carotid artery were then exposed by an incision above the left clavicle. A Terumo Soft 0.36 (Terumo, Europe) guidewire was inserted through the left common carotid artery and extracted by a loop in the left groin. The guidewire was a marker for a fenestrated stent graft on the brachiocephalic trunk. On the same side of the groin a pigtail catheter was placed in the ascending aorta. Additionally, an electrode for “rapid pacing” was placed in the right ventricle through a 7F port located in the left femoral vein. Before the introduction of PMEG a 7F port was inserted into the left subclavian artery (LSA) and a Vanchi1 (COOK Medical, Bloomington, USA) diagnostic catheter was left in it, marking the departure of the LSA. Before insertion of the stent graft a full dose of heparin was injected (100 mg under ACT control, the recommended range of which should not exceeded 250 s). A LunderQuist double curved guide (COOK Medical, Bloomington, USA) was inserted into the left ventricle from the right groin and was loaded with the PMEG delivery system. The PMEG delivery system was positioned according to the guidewire coming from the left carotid artery. The markers “8” were located on the greater curvature. The stent graft was released rapidly. Initially we noticed a slight rotation that caused lowering of right limb blood pressure without lowering NIRS (near infrared spectroscopy, EleVision IR Platform, Medtronic). It was corrected by pushing the stent graft away from the greater curvature by a cannula inserted deeper from a left carotid artery. Then the fenestrations for the brachiocephalic trunk were cannulated from the right femoral artery and the left subclavian artery. In order to speed up the procedure two teams performed this activity. Finally, the appropriate shirts were placed into the stent graft fenestrations and packed with stent grafts. For the brachiocephalic trunk a VBX 9 × 80 mm stent graft was chosen (GORE, Flagstaff, USA), followed by a Lifestream 8 × 59 mm (Bard Medical, USA) placed in the left subclavian artery fenestration. The stent graft in the LSA was first deployed and flared inside its aortic part. Selective angiography confirmed its patency and lack of leakage. The left artery guide wire was then removed and left carotid-subclavian bypass was made using an 8 mm ringed prothesis. The proximal segment of the left carotid artery was blindly occluded to cut off the retrograde inflow to the pseudoaneurysm. After unclamping the bypass, blood began to flow to the left hemisphere through fenestrated stent graft and the LCCA-LSA bypass. Finally, the stent graft into the BCT was deployed and flared. Selective angiography confirmed it patency. Additional ballooning of the initial section of the VBX inside the bovine arch was performed to achieve good positioning of the VBX stent graft to the artery wall. Due to this, the inflow of blood to the right hemisphere of the brain and the right hand was restored, omitting the aneurysm. In the final angiography, the patency of the cerebral vessels and the reconstruction of the aortic arch continuity were confirmed. The delivery system was removed and the femoral arteries were occluded with the ProGlide system. The effect of heparin was reversed with protamine sulfate 1 : 1.5 and the ACT control was performed, confirming its normalization. Finally, a wound on the neck was sutured, leaving a Redon drain. The procedure (including the time of graft modification) was completed in approximately 4.5 h. There was no significant decrease of hemoglobin level after the procedure (Figure 2).
Figure 2
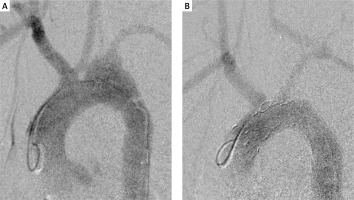
A – Initial angiography exposed aortic damage and pseudoaneurysm. B – Final angiography showed restored continuity of the aortic arch and appropriate inflow to the cerebral vessels
The patient also underwent revision of the abdominal organ injuries with some reconstructions the next day. Orthopedic surgery of the upper and left lower limb was also performed with external stabilization after 2 days. Due to damage of large muscle masses and myoglobinuria the patient developed acute renal failure in the following days and required renal replacement therapy. As a result of extensive trauma to the parenchyma of the liver and biliary tract the patient developed acute liver failure and died 8 days after aortic arch surgery.
Discussion
All aortic arch pathologies (zones 0-2) among young people should be treated with open surgery, because stent graft technology is “too young” to predict long-term results. Only open treatment provides a permanent cure. The most common aortic injuries affect the descending aorta (zones 3 and 4) [1] and they can be easily treated with TEVAR. Multi-organ injuries with active bleeding from the parenchymal organs require intensive alignment of the coagulation system. Heparinization necessary for extracorporeal circulation and hypothermia also very seriously worsen the coagulation disorders. Even initially healthy patients might require massive transfusions of blood products including platelets, coagulation factors and cryoprecipitate after FET. A patient with active hemorrhage has no chance of surviving such additional trauma. Such location of the arch trauma can by treated less invasively by aortic arch debranching, but it still requires heparinization and sternotomy. Currently, there are no existing off-the-shelf arch devices (ready-made stent grafts that are suitable for everyone’s anatomy). All stent grafts on the market are customized and their production process takes 8 to 12 weeks. To simplify the endovascular procedure, parallel grafts are used in some centers. However, in the present case no kind of parallel graft (chimney, periscope) was considered due to a gutter leakage, because they are not recommended in such cases as a primary treatment option. It could be only considered as a bail-out in case of technical failure of the FEVAR, BEVAR or TEVAR. Additionally, in the present case a common departure from the innominate artery and left carotid artery would trigger a competition between the radial force of the two parallel stent grafts going through the narrow area, with difficult-to-predict consequences. Despite risks related to some heparinization (in a lower dose, but still ACT extension) and technical difficulties (modification of the stent graft requires experience, just as inside arch implantation requires an understanding of the graft’s behavior when passing through its curvature), the procedure was safely performed [3–5]. It shows that it is feasible in urgent cases.
Of course, technical success in such an extensive injury does not guarantee a good result early. Such extensive trauma generally reduces the chances for survival. However, the decision to start treatment was made due to the young age. Additionally, endovascular treatment in a young patient cannot guarantee a good long-term result. If the patient survived, he would face age-related aortic degeneration (increasing diameters and lengths) which could eventually lead to leakage. The patient would eventually need an FET operation. Moreover, the patient would stay in a life-long surveillance program (angio-CT yearly) and be exposed to a huge cumulative dose of radiation.








