Introduction
Staged palliative operations, including the Norwood, Glenn, and Fontan procedures, provide morbidity and mortality hazards [1]. Therefore, new solutions are being explored, including hybrid procedures to provide alternative therapeutic options in children expected to have suboptimal surgical outcomes and who are at high risk of cardiopulmonary bypass [1–3].
Hybrid procedures (HP) combine surgical and catheter-based interventions to maximize the survival rate, minimize perioperative complications, and provide a temporary solution for children with borderline left ventricle (LV) with a chance of biventricular completion in the future [4, 5]. The decision-making process always poses a dilemma regarding which option to choose in neonates with single ventricle (SV) physiology, including: 1) the Norwood procedure (with Blalock-Taussig shunt or Sano modification), 2) hybrid approach, 3) bilateral pulmonary artery banding (PAB) with PGE1 infusion as a bridge to comprehensive stage 2 or heart transplantation, and 4) compassionate care with discontinuation of aggressive therapy, especially in children with chromosomal abnormalities [6, 7].
According to the literature, each option is associated with high mortality, and the initial enthusiasm for HP has been diminishing. Therefore, we analyzed the merits and pitfalls of HP as a minimally invasive therapy that has space for improvement.
Aim
This study aims to assess hybrid procedures in neonates with complex left-sided obstructive lesions and duct-dependent systemic flow. By analyzing a single center’s experience, we identify critical procedural pitfalls and propose strategies to optimize perioperative management and improve long-term outcomes.
Material and methods
Data were collected from 40 patients diagnosed with SV physiology, left-sided obstructive lesions (LSOL) and duct-dependent systemic flow (DDSF) between the period 2019–2024. Of these, 12 patients were scheduled for HP, 16 for a Norwood operation (6 with Blalock-Taussig anastomosis from the brachiocephalic artery to the right pulmonary artery and 10 with Sano modification), and 12 for reconstruction of the aortic arch and bilateral PAB. A retrospective analysis was performed in 12 children following HP, according to hospital standards. Therefore, ethical committee approval was not required.
The inclusion criteria were as follows:
infants with SV physiology and DDSF requiring PGE1 infusion,
HP as initial therapy with bilateral PAB, ductus arteriosus (DA) stenting, balloon atrial septostomy (BAS) or septectomy in cases of restrictive foramen ovale (FO) or intact atrial septum (IAS).
The exclusion criteria were as follows:
Results
Twelve neonates (7 boys and 5 girls) were treated with HP. Demographic data and stepwise therapy with re-interventions and mortality are presented in Table I. Routine diagnostic work-up with prenatal ultrasound was performed in 10 patients and confirmed by postnatal transthoracic echocardiography (TTE). Two neonates had no prenatal diagnosis and developed cardiogenic shock after hospital discharge.
Table I
Demographic data of the patients with type of heart defect and procedures followed by hybrid approach
[i] AVS – critical aortic valvar stenosis, BAS – balloon atrial septostomy, BivSol – biventricular solution, CATH – catheterization, DA – ductus arteriosus stent implantation, DORV – double outlet right ventricle, ECMO – extracorporeal membrane oxygenation, HLHS – hypoplastic left heart syndrome, HLHC – hypoplastic left heart complex, LPA – left pulmonary artery stenting, MV – artificial mitral valve implantation, PAB – pulmonary artery banding, TV – tricuspid valve, unb. AVSD – unbalanced atrioventricular septal defect.
The main indications for HP were poor general condition with severe cardiac compromise (n = 12), multi-organ dysfunction (n = 12), extremely hypoplastic ascending aorta (n = 3) (Figure 1) with a diameter of less than 3 mm, and low body weight (below 3 kg in 3 neonates). The procedures were performed at an average age of 8.6, SD 9.2 days, although in 4 children urgent interventions were necessary after birth due to the critical condition. The mean body weight at the operation was 3.3, SD 0.3 kg.
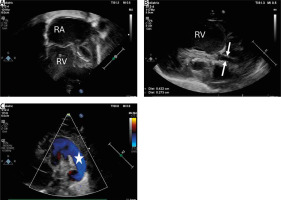
Figure 1
Transthoracic echocardiography (TTE) showing hypoplastic left heart syndrome. A – TTE. 2DE. Apical 4 chamber view showing dilated right atrium and right ventricle and extremely hypoplastic left ventricle with fibroelastosis. B – Parasternal longitudinal axis. Aortic valve atresia with extremely hypoplastic ascending aorta (2.7 mm) (white arrows) and bulbus of the aorta (4.2 mm). C – Parasternal short axis view with color Doppler flow showing wide patent arterial duct (white star) with right-to-left shunting
RA – right atrium, RV – right ventricle.
Hybrid procedures – percutaneous interventions
Initial percutaneous intervention, as the first stage of HP, was performed using a 6 or 7F catheter in the right femoral vein in 6 patients due to: 1) restrictive FO with BAS (n = 4), 2) intact atrial septum with septal perforation (n = 1), 3) critical aortic valve stenosis (AVS) with balloon aortic valvuloplasty (n = 2), 4) DA severe stenosis despite PGE1 infusion with Palmaz Genesis 9 mm/18 mm and 10 mm/19 mm stent deployment (n = 2). Patient 10 had a stent implanted 7 days after PAB (Figure 2).
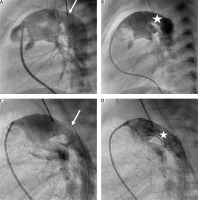
Figure 2
Percutaneous arterial duct stent implantation. A and B in Patient 11 – Pulmonary artery angiography in lateral view showing tight pulmonary bands, mild narrowing (6.5 mm) of the arterial duct (white arrow) at the pulmonary end, and aneurysmal dilation (11.5 mm) at the aortic end. B – Pulmonary angiography following Palmaz Genesis 10 mm/19 mm stent deployment with unobturated flow (white star) and tight pulmonary bands. C and D in Patient 10 – Pulmonary artery angiography in a patient with severely stenotic arterial duct (3.5 mm) at the pulmonary end and dilation (7 mm) at the aortic end. Pulmonary arteries without bands. D – Pulmonary artery angiography following Palmaz Genesis 9 mm/18 mm stent deployment providing effective flow into the descending aorta
Hybrid procedures – surgical interventions
In 7 patients, HP was performed without aortopulmonary bypass. Short-term cross-clamp circulation was necessary in 5 patients who required septectomy (n = 5) and simultaneous aortic valvulotomy (n = 1). In all patients, bilateral PAB was performed with 3 mm rings made of 3.5 mm Gore-Tex graft, adjusting the tightness to a SaO2 level of more than 80%.
Preceding a stent implantation, a 7F sheath was inserted into the pulmonary artery and subsequent angiography showed the size and length of the DA and made it possible to establish a landing zone which started at the origin of the left pulmonary artery (LPA). Balloon expandable stents (Palmaz Genesis) ranging in size from 7 mm/12 mm to 10 mm/19 mm were deployed into the arterial duct. Unfortunately, if the stent was too short (less than 15 mm) or too narrow (less than 8 mm), the procedure was complicated with stent dislodgment or DA proximal or distal stenosis with the need for additional interventions. Another crucial problem was obturated retrograde flow into the aortic arch due to the stent or isthmus stenosis limiting the coronary and cerebral flows (Figures 3–5).
Figure 3
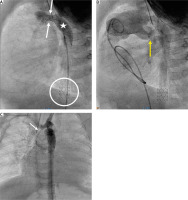
Percutaneous arterial duct stent deployment and banding balloon plasty. A – Pulmonary artery angiography in lateral view in patient 6 following Palmaz Genesis 8 mm/12 mm stent deployment into the arterial duct (white star) with proximal duct stenosis (white arrow). B – Left pulmonary artery band balloon plasty with an indentation at the tight band (yellow arrow). White stars indicate 2 wide stents implanted into the stenotic arterial duct. C – Right pulmonary artery band balloon plasty with an indentation at the tight band (yellow arrow)
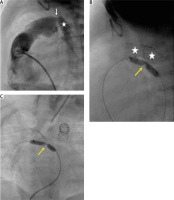
Figure 4
Arterial duct stent implantation during hybrid procedure. A – Pulmonary artery angiography during hybrid procedure performed through the sheath introduced into the artery. Tight bands over the pulmonary branches (yellow arrow). Wide arterial duct (white star). B – A stent Palmaz Genesis 8 mm/12 mm (white arrow) implanted into the proximal part of the arterial duct. Distal arterial duct stenosis (red arrow). C – Arterial duct angiography following second Palmaz Genesis 8 mm/12 mm stent deployment (dash line) and wide antegrade flow into the descending aorta and obstructed retrograde flow into the aortic arch through the stenotic isthmus (white arrow)
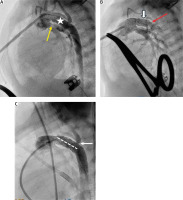
Figure 5
Arterial duct stent dislodgment into the descending aorta. A, B – Pulmonary artery angiography in lateral view showing proximal arterial duct stenosis (white arrows). A stent Palmaz Genesis 8 mm/15 mm deployed in the distal arterial duct. White circle indicates a dislodged stent Palmaz Genesis 7 mm/12 mm following re-dilation. B – Tight pulmonary arteries band (yellow arrow). C – Aortic arch angiography showing extremely hypoplastic ascending aorta (white arrow)
In the postoperative course, extracorporeal membrane oxygenation (ECMO) was necessary in 2 patients following HP. Patient 6 with HLHS and ineffective BAS complicated with TV damage survived after the reoperation of severely regurgitant TV. Patient 11 with critical aortic stenosis presented with symptoms of insufficient coronary perfusion following aortic valvulotomy and DA stent implantation and died despite conversion to the Norwood procedure.
Follow-up
The follow-up of the whole group was x = 2.4 years, SD 1.7. Early mortality (defined as death within 30 postoperative days) was 8.3%. Late mortality was 16.6%, at a mean age of 178.0, SD 112.0 days due to progressive cardiopulmonary compromise with severe TVR and RV dysfunction. One patient died after comprehensive stage 2. Two patients underwent a Fontan operation with fenestration and two others are still waiting. Two children after a Glenn operation were disqualified from further palliative procedures due to pulmonary vein stenosis (n = 1), and multiorgan dysfunction with chronic respiratory failure (n = 1).
Percutaneous re-interventions
Interstage catheterization was performed in 7 patients for hemodynamic evaluation, PAB re-dilation (n = 3) due to progressive hypoxia (SaO2 < 70%), BAS with transhepatic access due to FO restriction (n = 1), additional stent implantation into the DA (Palmaz Genesis 8 mm/15 mm) (n = 1) and DA stent re-dilation (n = 1) due to significant stenosis.
Comprehensive stage 2
Comprehensive stage 2 with Norwood and Glenn procedures were performed in 7 patients at the age of x = 6.9 months (SD 1.6 months). During the operation the aortic arch was reconstructed altogether with bidirectional Glenn anastomosis and pulmonary branches plasty. The Palmaz Genesis 8 mm/15 mm stent was implanted into the stenotic or hypoplastic left pulmonary artery in 3 children. The azygos vein was embolized routinely to prevent the blood steal phenomenon and progressive cyanosis.
In 2 children (patients 2 and 5) a biventricular solution was implemented with the need for implantation of an artificial mitral valve (SJM Masters Series 17 mm valve and Regent bi-leaflet Masters Series).
Discussion
Left-sided obstructive and hypoplastic lesions represent a broad spectrum of congenital heart defects requiring palliative (temporary or definite) operations [8–10].
Palliative procedures provide a staged approach in single ventricle physiology or a temporary solution before biventricular conversion. HP provide an alternative option for critically ill neonates for hemodynamic stabilization and as a bridge to stage 1 or even comprehensive stage 2 [10, 11]. They combine catheter-based techniques with surgical procedures, resulting in better overall outcomes when the cross-clamp circulation is associated with high surgical risk.
HP may provide significantly lower mortality compared to the Norwood operation if performed by an experienced heart team. However, in the postoperative period progressive hypoxia due to tight pulmonary bands and narrowing of the DA stent require additional interventions with early conversion to stage 1.
Technical challenges during percutaneous or hybrid interventions remain a major problem in palliative therapy [12, 13]. This can have a direct impact on patient mortality and morbidity. In order to improve treatment results, a number of challenges to overcome have been identified, and potential solutions are indicated.
Heart team meetings
Prenatal diagnosis provides a chance for early heart team evaluation of the most optimal surgical strategy. Prenatal risk assessment coupled with early postnatal interventions is essential for optimizing outcomes and preventing severe postoperative complications.
Throughout this process, ongoing collaboration through heart team meetings has been indispensable. In our cohort, early postnatal TTE was performed in the delivery room or intensive care unit and provided critical information for prompt initiation of therapeutic strategies. Newborns with low birth weight (< 2.5 kg), severe cardiopulmonary compromise, IAS or extremely restrictive FO required early intervention to prevent multi-organ failure and adverse outcomes. In the management of HP in newborns with SV physiology and DDSF, several insights that could help future clinicians navigate common challenges and minimize complications have been identified and highlighted in this paper. Our experience emphasizes that thorough preparatory steps and careful intra-procedural adjustments are vital to achieve optimal outcomes in these high-risk patients.
Hybrid procedures
HP include 3 crucial interventions: 1) creation of a wide interatrial communication with BAS or surgical septectomy, 2) DA stent implantation and 3) bilateral PAB [12].
Balloon septostomy versus surgical septectomy
Percutaneous BAS is an initial part of HP. It is performed under fluoroscopy and with TTE guidance with a multipurpose catheter introduced into the left atrium over a guidewire and exchanged into a 13.5 mm Z5 NuMED septostomy balloon, which is inflated and pulled back to the right atrium even a few times before TTE confirms decompression of the left atrium and a wide interatrial communication. This allows the HP to be performed off-pump without the need for septectomy. However, BAS may be challenging, time consuming and insufficient, especially in critically ill patients with unfavorable anatomy of the interatrial septum, highly restrictive FO or IAS. In these cases, surgical septectomy seems to be a preferred option due to the relatively low success rate of septum perforation and high risk of myocardial perforation with tamponade. This approach spares patients from prolonged, destabilizing interventions and provides quicker relief for urgent cases.
Hybrid stent deployment
Performing a preliminary pulmonary angiography in lateral view as the initial imaging allows us to assess the architecture of the DA, such as its curvature, width, and length as well as the presence of aortic coarctation [11, 13, 14]. By understanding these nuances, we are better equipped to anticipate and manage any potential stent migration or impairment of retrograde flow into the aortic arch at the level of the isthmus. Selecting the appropriate stent size, length and landing zone is also crucial, as we have observed that narrower (less than 8 mm) and shorter (less than 15 mm) stents have higher risk of migration into the descending aorta or pulmonary trunk (Figure 4). In the case of stent displacement, re-dilating it above the celiac trunk can restore flow, avoiding further complications. Additionally, managing a wide DA requires halting PGE1 infusion before the procedure and selecting the widest expandable stent (usually 10 mm). This minimizes the risk of stent migration. To confirm these adjustments, a second angiography serves as a key checkpoint, ensuring stent patency and confirming that sufficient flow is maintained into the descending aorta as well as retrograde flow into the aortic arch [15, 16]. DA stent deployment is essential for maintaining sufficient systemic flow. If the stent is implanted too deeply into the descending aorta, it may be difficult to excise it during stage 2, while the aortic arch is reconstructed. Additionally, retrograde flow through the stent may restrict coronary and cerebral blood perfusion.
Bilateral pulmonary arteries bands
Adequate pulmonary flow can be achieved by precise adjustment of the bilateral PAB with Gore-Tex rings of varying diameters based on the patient’s weight. Effective PAB reduces pulmonary blood flow, and helps to protect the lungs from over-circulation [17]. Following the recommendations of Galantowicz [18] and our experience, we suggest using narrow 2–3 mm long Gore-Tex rings with a diameter of 2–3 mm for children weighing less than 3 kg, and 3–3.5 mm rings for higher body weight. SaO2 level serves as the most important indicator of optimal band tightness. In some cases, the right pulmonary artery band can be tightened further (up to 2 mm) to achieve appropriate SaO2 (approximately 80%).
Coronary perfusion
Insufficient coronary and cerebral perfusion may be life-threatening in children with HLHS and extremely hypoplastic ascending aorta following HP [16]. For these patients, ECMO and conversion to Norwood circulation seem to be the only viable options to prevent coronary hypoperfusion and sudden cardiac arrest. During the interstage period, echocardiographic features remain crucial for monitoring hemodynamic balance, which is highly correlated with patient follow-up.
Progressive hypoxia in follow-up
Hypoxia can initiate neo-angiogenesis, which leads to the development of systemic-pulmonary anastomoses. These newly formed collaterals provide additional pulmonary blood flow despite the presence of tight PAB. In some cases, the collateral circulation may be sufficient to alleviate hypoxia temporarily, reducing the need for further interventions.
Follow-up management also benefits from targeted interventions, such as PAB re-dilation with a 3 or 4 mm balloon catheter, especially for infants experiencing progressive cyanosis. Neo-angiogenesis can foster development of collaterals to maintain sufficient SaO2. These anastomoses may require embolization in patients following the Glenn operation, particularly if superior vena cava syndrome develops. The timing of comprehensive stage 2 remains a matter of ongoing debate. Progressive hypoxia, often due to overly tight PAB, may necessitate early transition to stage 2, typically performed around 4 to 6 months of age.
Follow-up
The mortality following HP depends on patient selection and the experience of the heart team. Table II presents the comparison of mortality ranging from 0 to 50% in children operated on with HP. It is controversial as to whether HP provide a lower mortality rate compared with the Norwood procedure. Karamlou et al. reported that HP may have lower mortality (16% vs. 43%) in centers with high hybrid use. On the other hand, patients with HP usually have higher preoperative risk factors, which may explain higher in-hospital mortality in some centers (30% vs. 16%, p < 0.01) [19]. Additional reinterventions and reoperations were necessary in both groups [20]. Furthermore, the mortality rates between HP and Norwood patients tend to equalize following the comprehensive stage 2 requiring aortic arch reconstruction, Glenn anastomosis, pulmonary artery plasty, and DA stent resection. It introduces additional risks of postoperative complications, including pulmonary artery stenoses and the need for stenting. Anatomical modifications resulting from the initial hybrid procedure often increase the technical complexity of subsequent surgery, heightening the overall surgical risk. Thorough evaluation by a multidisciplinary cardiology team are critical for devising the most appropriate treatment strategy.
Table II
Comparison of mortality in patients following hybrid procedures
| Author | Year | Patients (n) | Birth weight, mean [kg] | In-hospital mortality (n/%) |
|---|---|---|---|---|
| Bitar F, et al. [3] | 2024 | 8 | 3.3 | 0/0 |
| Erek E, et al. [7] | 2020 | 65 | 3 | 27/41.5 |
| Wilder TJ, et al. [6] | 2017 | 110 | 2.9 | 44/40 |
| Brescia AA, et al. [4] | 2014 | 24 | 2.6 | 7/29 |
| Baba K, et al. [9] | 2012 | 32 | – | 2/6 |
| Honjo O, et al. [20] | 2009 | 19 | 3.3 | 4/21 |
| Pizarro C, et al. [1] | 2008 | 14 | 2.6 | 7/50 |
| Galantowicz M, et al. [18] | 2008 | 40 | 3.1 | 7/17.5 |
Limitations
This study possesses several potential limitations that should be acknowledged. First, it is a retrospective review conducted at a single institution, which may introduce selection and observational biases. The small sample size (n = 12) limits the applicability of the findings to larger or more diverse populations and restricts the data analysis by separation into subgroups. The heterogeneity of congenital heart defects differs between patients, which may influence the outcomes observed. In addition, lack of a standardized follow-up protocol could have affected interstage monitoring and timing of reinterventions.
Thus, future multicenter, prospective studies with larger sample sizes and standardized protocols are needed to validate these observations and optimize hybrid procedures for high-risk neonates.
Conclusions
Managing newborns with SV and DDSF requires precise techniques of the HP to mitigate common pitfalls. Prenatal diagnosis and heart team meetings enable early identification of candidates for HP. For critically ill patients, opting for septectomy over balloon septostomy, combined with simultaneous HP, can prevent destabilization. Conducting initial pulmonary artery angiography to assess the landing zone and precisely selecting the stent size and length may improve procedural success. Accurate bilateral PAB ensures a balance between systemic and pulmonary circulations. Postoperative hypoxia may require PAB adjustment with interventional balloon plasty, potentially delaying comprehensive stage 2 surgery. The refinements in hybrid techniques emphasize the importance of comprehensive planning and reduce the mortality and unexpected complications.






