Summary
The inflammatory activation presented as the plasma extracellular vesicle (EV) count from erythrocytes, leukocytes, and platelets decreases after surgical revascularization. Arterial revascularization of the left descending artery (LAD) reduces the number of leukocyte-derived EVs. Left or right internal mammary artery anastomosis to the LAD significantly reduces the total plasma EV count.The EV count changes are more profound in arterial than in mixed arterio-venous grafting.
Introduction
In multivessel coronary disease, intervention therapy is recommended to improve symptoms and survival [1]. In recent trials, conservative therapy for chronic coronary syndrome has been proposed [2]. According to Doenst et al. [3], the optimal result regarding survival is still attributed to surgical coronary revascularization in patients with triple vessel disease. For long-term prognosis, demographic and clinical factors followed by surgical techniques are crucial [4, 5].
In recent years, a successful trend toward coronary disease risk factor modification has been observed regarding pharmacotherapy, smoking cessation, and active lifestyle promotion [6]. Modifying inflammatory activation, which is postulated as one of the risk factors for coronary atherosclerosis [7], may effectively reduce primary endpoints [8] in patients with coronary artery disease.
The relationship between coronary culprit lesion progression and inflammatory indices has already been presented [9], including simple inflammatory markers [10] and cytokines [11] but also novel markers such as extracellular vesicles (EVs) [9]. The plasma EV count increases 2- to 3-fold in patients with coronary artery disease [10, 11]. Through their extreme heterogeneity in surface markers and cargo, EVs as cell-derived membrane-enclosed particles play various modulatory roles in intercellular communication [12].
Surgical revascularization allows the achievement of excellent long-term results, mostly due to left internal mammary artery patency rates [13]. The two techniques, on-pump, and off-pump surgery, present satisfactory long-term results [14]. The number of arterial anastomoses is claimed to be an independent long-term predictor of revascularization completeness and the number of performed grafts [15].
The surgical intervention activates inflammatory reactions, though in off-pump surgery, a technique-related protective effect is postulated [16]. The inflammatory activation of endothelial cells is related to venous graft disease [17]. Perioperative inflammatory activation is believed to influence patients’ prognosis [18].
Aim
We aimed to evaluate factors associated with perioperative EV plasma count and search for a relation between formation changes and performed revascularization. Patients were divided into two groups related to arterial and combined (arterial and venous) revascularization. The measurements of graft blood flow to target vessels in relation to the number of plasma EV changes were investigated.
Material and methods
Patients
Forty-four (33.75%) patients with a median age of 65 (58–69) years who underwent surgical revascularization due to multivessel coronary disease in 2021 were included in the study. Arterial hypertension was diagnosed in 35 (80%) patients, followed by hypercholesterolemia in 28 (64%), and diabetes mellitus and peripheral artery disease in 14 each (32%). Nineteen (43%) patients had a history of myocardial infarction. Chronic pulmonary disease was found in 2 (5%). The mean body mass index was 29.3 (25.2–33.1). Patients were divided into two groups related to arterial and combined (arterial and venous) revascularization.
Surgical technique
All procedures were performed through median sternotomy after heparinization to achieve activated clotting time (ACT) above 350 s. The procedures were performed as beating heart surgery. The heart was elevated using a deep pericardial suture. The anastomosis was performed after local stabilization with an Octopus III device (Medtronic, USA), and after artery incision, intraluminal shunts were used to obtain a bloodless surgical field. Continuous 7-0 monofilament suture was used for the distal and 6-0 for the proximal anastomosis. The proximal venous grafts were sutured to the ascending aorta after partial clamping.
EV measurements
EVs were measured preoperatively (hospital admission) and on the 1st and 3rd postoperative days. Blood was collected into 4.9 ml K3-EDTA plastic probes (S-Monovette, Sarstedt, Nümbrecht, Germany).
The EV isolation was performed in line with previously proposed protocols [19]. Blood was collected in 10 ml 0.109 mol/l citrated plastic tubes and 4.9 mL K3-EDTA plastic probes (Nümbrecht, Germany). Within 30 min from blood collection, platelet-depleted plasma was prepared by double centrifugation with the following centrifugation parameters: 2,500 g, 15 min, 20°C, acceleration speed 1, no brake. After the second centrifugation step, platelet-depleted plasma was mixed by pipetting, transferred to 1.5 ml Eppendorf tubes (Thermo Fisher Scientific, MA, USA), and stored at –80°C until analyzed. Prior to analysis, samples were transported to the Vesicle Observation Centre, Amsterdam, University Medical Centers (UMC), The Netherlands.
EV counts were measured by flow cytometry (A60-Micro, Apogee Flow Systems, Hertfordshire, UK) at a count rate of less than 3000 events/s during 120 s at a flow rate of 3.01 μl per min. We measured concentrations of EVs from erythrocytes (CD235a+), leukocytes (CD45+) and platelets (CD61+).
The EV isolation was performed according to previous protocols.
Graft blood flow measurements
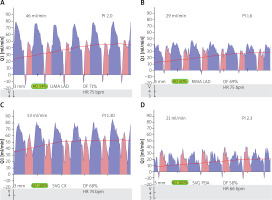
The graft blood flow measurements were performed using ultrasonography equipped with a linear 6.5 MHz transducer (Verify Q, USA) after the second dose of protamine when the patient was hemodynamically stable. The sites of graft flow assessment were as follows: left and right internal mammary arteries (LIMA and RIMA) were approximately 10 cm from the origin from subclavian arteries, whereas radial artery (RA) or saphenous grafts (SVBG) were measured 5 cm from the proximal anastomosis to the ascending aorta. The blood flow measurements were followed by determination of the pulsation index (PI) to confirm a satisfactory result.
Statistical analysis
All continuous data were presented as the median and interquartile range, Me (Q1–Q3), because data did not follow a normal distribution (Shapiro-Wilk test). A non-parametric test (Mann-Whitney) was used to compare the continuous variables between arterial and arterio-venous groups. The Friedman test was used to analyze the EV plasma concentration changes, followed by the post-hoc Dunn’s test. A linear regression model was used to assess the relationship between graft blood flow and EV plasma count changes. The results were presented as the coefficient (Coeff.) and 95% confidence interval (95% CI). The statistical analysis and graphical visualization were performed using the MedCalc statistical package, MedCalc1 Statistical Software version 20.010 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021).
Results
There were no intra- or postoperative deaths in the study group. The median hospitalization time was 10 (9–15) days, including a postoperative stay of 7 (7–9) days. The mean number of performed grafts was 2.3 (0.6).
The serum EV counts, followed by laboratory results, were compared in arterial and combined (arterio-venous) groups and are presented in Table I.
Table I
Intraoperative characteristics of studied groups
[i] CK-MB – creatine kinase – myocardial, Cx – circumplex artery, LAD – left descending artery, LIMA – left internal mammary artery, LRA – left radial artery, RCA – right coronary artery, RIMA – right internal mammary artery, SVBG1 – saphenous vein bypass graft 1, SVBG2 – saphenous vein bypass graft 2.
Blood flow measurements
The blood flow measurements in performed anastomosis were characterized by flow measurements (ml/min) and pulsatility index (PI) in relation to heart rate (HR) and Doppler flowmetry (DF) (Figure 1).
Plasma EV number changes within groups
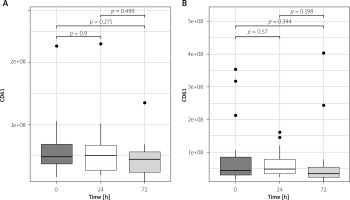
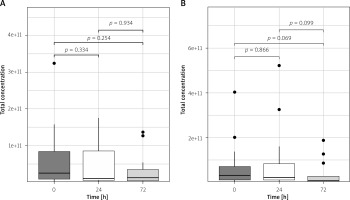
There were no significant differences in total plasma number of EVs between pre-operative and post-operative measurements (p > 0.25 for all) in the arterial revascularization group and in combined (arterial and venous) revascularization (p > 0.06 for all; Figures 2 A, B).
Figure 2
Total plasma EV concentration changes in arterial (A) and arterio-venous (B) groups in perioperative period
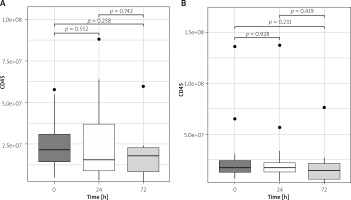
There were no significant differences in leukocyte-derived plasma EV count between pre-operative and post-operative measurements in the arterial revascularization group (p > 0.24 for all) and in the combined (arterial and venous) revascularization group (p > 0.23 for all; Figures 3 A, B).
Figure 3
Plasma CD45 EV concentration changes in arterial (A) and arterio-venous (B) groups in perioperative period
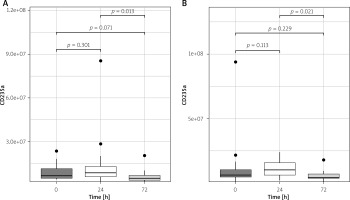
Plasma erythrocyte-derived EV numbers were lower on the 3rd post-operative day, compared to 1st post-operative day both in the arterial revascularization group (p = 0.013) and in the combined (arterial and venous) revascularization group (p = 0.031; Figures 4 A, B).
Figure 4
Plasma CD235 EV concentration changes in arterial (A) and arterio-venous (B) groups in perioperative period
There were no significant differences in platelet-derived plasma EV counts between pre-operative and post-operative measurements in the arterial revascularization group (p > 0.27 for all) and in the combined (arterial and venous) revascularization group (p > 0.19 for all; Figures 5 A, B).
Comparison of EV plasma counts between arterial and combined revascularization groups
The total plasma EV numbers were lower in the arterial revascularization group, compared to the combined group on the 1st postoperative day (p = 0.014). No other significant differences were found between the groups (Table II).
Table II
Perioperative plasma EV concentrations in studied groups
| eVs concentration [ml–1] | Arterial group (n = 23) | Arterio-venous group (n = 19) | P-value |
|---|---|---|---|
| Total EV concentration (e+10): | |||
| Before surgery (0), median (Q1–Q3) | 2.47 (0.84–9.23) | 2.93 (0.91–7.46) | 0.860 |
| 24 h after surgery (1), median (Q1–Q3) | 0.96 (0.55–8.56) | 1.93 (0.91–8.95) | 0.255 |
| 72 h after surgery (3), median (Q1–Q3) | 1.23 (0.40–3.48) | 0.65 (0.57–2.64) | 0.942 |
| 0-1 difference, median (Q1–Q3) | 0.66 (–0.11–1.46) | –1.02 (–4.20–0.34) | 0.015* |
| 0-3 difference, median (Q1– Q3) | 1.78 (0.74–4.03) | 1.42 (0.21–4.21) | 0.963 |
| Leukocyte-derived EVs (CD45+) (e+07): | |||
| Before surgery (0), median (Q1–Q3) | 2.15 (1.21–3.36) | 1.69 (1.22–2.41) | 0.480 |
| 24 h after surgery (1), median (Q1–Q3) | 1.54 (0.88–3.70) | 1.69 (1.27–2.25) | 0.987 |
| 72 h after surgery (3), median (Q1–Q3) | 1.79 (0.84–2.29) | 1.43 (0.58–2.17) | 0.680 |
| 0-1 difference, median (Q1–Q3) | 0.49 (–0.75–1.29) | –0.43 (–0.82–0.48) | 0.228 |
| 0-3 difference, median (Q1– Q3) | 0.89 (0.44–1.92) | 0.32 (– 0.17–0.56) | 0.077 |
| Erythrocyte-derived EVs (CD235a+) (e+07): | |||
| Before surgery (0), median (Q1–Q3) | 0.66 (0.48–1.17) | 0.64 (0.46–1.14) | 0.723 |
| 24 h after surgery (1), median (Q1–Q3) | 0.84 (0.60–1.32) | 1.04 (0.63–1.57) | 0.805 |
| 72 h after surgery (3), median (Q1–Q3) | 0.47 (0.38–0.68) | 0.48 (0.39–0.70) | 0.865 |
| 0-1 difference, median (Q1–Q3) | –0.28 (–0.56–0.26) | –0.52 (–0.75–(–)0.27) | 0.283 |
| 0-3 difference, median (Q1–Q3) | 0.11 (–0.08–0.45) | –0.01 (–0.22–0.1) | 0.055 |
| Platelet-derived EVs (CD61+) (e+07): | |||
| before surgery (0), median (Q1–Q3) | 4.80 (3.47–7.09) | 4.36 (2.94–8.52) | 0.631 |
| 24 h after surgery (1), median (Q1–Q3) | 4.99 (2.60–6.60) | 4.84 (3.44–7.80) | 0.558 |
| 72 h after surgery (3), median (Q1–Q3) | 4.39 (2.30–5.60) | 3.49 (2.34–5.37) | 0.903 |
| 0-1 difference, median (Q1–Q3) | 0.60 (– 1.28–2.35) | –0.35 (–5.55–(–)0.12) | 0.203 |
| 0-3 difference, median (Q1– Q3) | 1.29 (–0.08–3.08) | –0.07 (–2.05–1.70) | 0.235 |
Coronary artery bypass blood flow parameters and EV plasma count
A regression analysis between blood flow parameters and EV plasma number was performed. The graft blood flow and EV count per unit changes were compared, and significant results are presented in Table III.
Table III
Perioperative plasma EV concentrations in studied groups
| eVs | Grafts | Coeff. | 95% CI | P-value |
|---|---|---|---|---|
| Total 0-3 | Arterial revascularization: RIMA flow to LAD LIMA flow Cx | –2.36e+09 4.47e+09 | –4.38e+9–(–)3.74e+08 3.74e+07–8.91e+09 | 0.028* 0.049* |
| Total 0-1 | Arterio-venous revascularization: LIMA flow to LAD | –6.97e+09 | –1.22e+9–(–)1.74e+09 | 0.015* |
| CD45 0-1 | Arterio-venous revascularization: LIMA flow to LAD | –1.67e+07 | –3.04e+7–(–)0.34e+07 | 0.020* |
Discussion
EVs refer to a heterogeneous group of un-replicable cell-derived circulating particles that are delimited by a lipid bilayer [20]. They are characterized by size and divided into small (diameter below 200 nm) and large (above 200 nm) or by density as low, medium, and high or by biochemical composition. Apart from their complexity, EVs serve diverse and important functions and developed as potential biomarkers, indicating their therapeutic applications [21]. The main findings of the study are that plasma erythrocyte-derived EV numbers were lower on the 3rd post-operative day, compared to the 1st post-operative day both in the arterial and combined (arterial and venous) revascularization group; total plasma EV counts were lower in the arterial revascularization group, compared to the combined group on the 1st postoperative day, and there was a significant correlation between leukocyte-derived EV decrease and left descending artery anastomosis.
Total plasma EV numbers were lower in the arterial revascularization group compared to the combined group on the 1st postoperative day, suggesting the important role of arterial revascularization. The arterial conduits are claimed to be related to better long-term results [22, 23] that are related to patency rates [24, 25], but according to our study, they may also play an important role in inflammatory status measured by plasma EV number.
Linear regression analysis revealed a significant relation between left descending artery revascularization and leukocyte-derived extracellular vesicles in the applied grafts. The results of our study indicate the significance of left descending artery (LAD) grafting as a main factor influencing leukocyte-derived EV count. Our analysis indicates the paramount role of LAD grafting in relation to EV changes.
We present the results of EV changes analysis related to surgical revascularization performed in the off-pump technique. Our analysis indicates that targeting the LAD during revascularization is of primary importance. The long-term patency results of LIMA-LAD anastomosis are believed to play a key role in coronary artery bypass grafting results. Our analysis not only revealed an EV decrease on the 1st operative day after surgical revascularization but also showed, for the first time, a lymphocyte-derived CD45 decrease in relation to LAD grafting. Moreover, the downregulations of EV numbers are related to blood flow characteristics in performed grafts.
We present a novel perspective of the LAD’s importance, as the regression analysis pointed out the role of LAD revascularization in perioperative EV changes. We present the results of arterio-venous grafting, revealing the significant role of LIMA-LAD blood flow measurements in EV counts. Interestingly, in the arterial revascularization group, the RIMA-LAD anastomosis was found to be significant for a postoperative EV decrease. In our analysis, the role of LAD as a target vessel is essential for plasma EV number.
The groups were controlled for demographic and clinical factors to eliminate their influence on interpretation of the results. Moreover, the procedures were performed successfully without reported complications, and the postoperative myocardial injury markers were insignificant within and between groups.
The graft’s blood flow characteristics appeared to inversely interfere with EV count, suggesting degradation of EV formation secondary to improving myocardial blood supply. Elevated counts of extracellular vesicles have been observed in patients with peripheral arterial disease [26]. The release of EVs from the ischemic myocardium has already been described by D’Ascenzo et al. [27]. The protective effect of EV release in anesthetized patients against hypoxia-induced ischemia was reported by Abel et al. [28].
Our analysis did not find significant differences between the groups related to EV count perioperatively except for significant differences between preoperative and 1st day total EV concentrations. The presented results indicate the trend of EVs decreasing after surgical revascularization. The decrease is more profound on 1st day compared to 3rd day results. The off-pump technique rules out the possible influence of cardiopulmonary bypass (CPB) presented by McVey et al. [29]. The study’s novelty is related to the decrease in perioperative number of EVs while off-pump coronary artery bypass grafting (OPCAB) is applied, contrary to extracorporeal circulation [30].
Interestingly, the total EV counts were significantly different when arterial revascularization was performed, contrary to combined arterio-venous grafting. The type of graft, not only the target vessel, may play a potential role in intercellular communications via EVs. One may hypothesize that incorporating venous material into arterial circulation plays a crucial role in formation of EVs.
CD45 is expressed on lymphocytes, and in our analysis, significant differences were observed in the arterial group, indicating its decrease in plasma. The regression analysis revealed the inverse relation between CD45 plasma count and blood flow measurements in LIMA grafts to LAD in arterio-venous grafting. On the first postoperative day, a significant decrease in plasma EV CD45 count was observed, indicating its possible role in interacting with environmental signals following surgical revascularization. The essential role of CD45 in signal transduction cellular response modulation was previously described [31]. CD45 up-regulation was found to be related to immune activation and myocardial muscle infiltration in animal studies in heart injuries [32–34].
Increased EV CD45 counts were observed on the first day following an acute episode of ischemic stroke [35]. In our results, the plasma EV concentration decreased, indicating the immediate protective effect of the performed grafting when applied by the OPCAB technique. This novel marker may indicate the protective effect of the revascularization technique as the CPB-related transient myocardial injury is postulated [36–38].
Angelillo-Scherrer [39] described the proinflammatory role of CD45-derived EVs as endothelial function modification and recruitment of inflammatory cells into the vascular wall. The novelty of our results is based on the down-regulation of leukocyte-derived (CD45) EVs related to graft blood flow characteristics. The negative relation between atrial graft blood flow parameters and EV count in the early postoperative period brings a new perspective of pathophysiological mechanisms that occur in coronary beds following surgical revascularization.
Nunez et al. [40] in their study found that a greater reduction of post-MI complication risk characterized patients with a low peripheral leucocyte count. The CD45 decrease in the arterial revascularization group may reveal a previously unknown relation with lymphocyte response to arterial grafting.
CD235 presents erythrocyte-derived extracellular vesicles that were postulated to possess a procoagulant role [41]. In our results, the increase of EV CD45 plasma count was followed by its statistically significant decrease.
The interplay between CD61 released from erythrocytes and off-pump surgery was found insignificant. This can be explained by surgical techniques that are contrary to hemodilution and mechanical injuries caused by CPB [42].
Our results highlight the significance of LAD revascularization. Not only does arterial grafting allow for satisfactory long-term results, but more importantly, based on the performed analysis, LAD revascularization provokes a significant change in the EV milieu. This pilot study has revealed a novel, unknown phenomenon related to coronary bed revascularization. The results did not support significant differences between LIMA-LAD and RIMA-LAD anastomosis related to blood flow parameters. Moreover, no significant differences between blood flow parameters and different coronary bed vessels were detected, favoring the conclusion that the blood flow and target vessels are not the key factors.
Study limitations. This was a single-center prospective study performed in off-pump patients referred for surgical interventions due to multivessel chronic coronary syndrome. Although periprocedural plasma EV changes as potential intercellular communication markers were observed, the precise pathophysiological processes remain unsolved. The study may indicate new research directions in patients undergoing original bypass surgery.