Introduction
Polycystic ovary syndrome (PCOS) is a highly prevalent endocrine-metabolic disorder in women of reproductive age. It is characterized by irregular menstruation/oligomenorrhoea, hirsutism, acne, oligo/anovulation, hyperandrogenaemia, polycystic ovaries on ultrasound, and infertility [1].
Infertility is a vital concern in women with PCOS. It is defined as the inability to achieve pregnancy after one year of unprotected intercourse. In patients 35 years of age or older, infertility is suspected after 6 months of unprotected intercourse rather than a year [2]. While several studies focused on the assessment of anovulation as an infertility cause in women with PCOS, available studies on the endometrium of these women are still limited.
Ovarian pathologies such as PCOS, and uterine pathologies such as polyps, are known to play a role in infertility, implantation failure, and recurrent miscarriages [3]. The incidence rate of endometrial polyps in women with infertility at reproductive age is about 15% [4].
In our study, we aimed to investigate the association between endometrial polyps and PCOS, in patients who were investigated for infertility after failure to achieve natural pregnancy after one year of regular unprotected intercourse. We looked at multiple risk factors associated with both polyps and PCOS, such as obesity and hyperoestrogenism, and performed an office hysteroscopy for PCOS patients with high suspicion of having endometrial polyps, to determine the prevalence of polyps in such patients.
Endometrial polyps are classified according to their location and size. Polyps less than 2 cm in size seem not to adversely affect the pregnancy rate [5]. However, uterotubal polyps show the greatest effect on infertility because they impede sperm transport, with a pregnancy rate after polypectomy of 57.4%, and the polyps with the least effect were in the anterior uterine wall with a pregnancy rate of (14.8%) after polypectomy [6].
Material and methods
We conducted a retrospective cohort study on a total of 250 patients with a confirmed diagnosis of a PCOS according to ESHRE/ASRM, Rotterdam criteria 2003. PCOS is diagnosed by 2 of the following 3 features: 1) oligo- or anovulation, 2) clinical and/or biochemical signs of hyperandrogenism, or 3) polycystic ovaries, and after the exclusion of related disorders: congenital adrenal hyperplasia, androgen-secreting tumours, Cushing’s syndrome [7]. These patients sought fertility advice at a specialized fertility clinic in Amman, Jordan between October 2019 and September 2021. All patients with abnormal basic fertility workup such as abnormal hysterosalpingogram findings, tubal factor infertility, severe male factor infertility, and anovulatory patients were later excluded from the study. The study was registered and approved by the Ethics Committee of Hashemite University (IRB 9/2/2021/2022)
Eighty patients out of the 250 were finally included in the study after a high suspicion of endometrial polyp by 2-D transvaginal ultrasound at the early follicular phase (day 2–3 of the menstrual cycle).
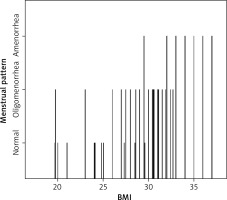
We asked the participants in great depth about their menstrual cycles pattern. Then we divided the menstrual pattern of these patients into 3 categories: normal, oligomenorrhoea, and amenorrhoea.
All patients underwent an office hysteroscopy: a rigid 0° view 2–9 mm diameter hysteroscope with working channel (Karl Storz), at the late follicular phase, after proper counselling, at the same menstrual cycle or another menstrual cycle to confirm the presence or absence of polyps, and to resect such polyps if present.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
All of the found endometrial polyps were resected at the same appointment using a cold knife (scissor), while women with submucosal fibroids were given another appointment, in which they had the fibroids resected by surgical hysteroscopy. All the collected data were later analysed using SPSS V.16.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study obtained Institutional Review Board approvel from Hashemite University (IRB 9/2/2021/2022).
Results
After excluding patients who did not meet the inclusion criteria, 80 patients were finally included. The ages of these women ranged from 19 to 38 years, with a mean of 28.39 years (Table 1). The body mass index (BMI) of the included women ranged from 19.6 to 37 with a mean of 29.68 (Table 1). The duration of infertility in women included in the study ranged from 1 to 6 years, with a mean of 3.2 years (Table 1). We divided the menstrual pattern of these patients into 3 categories: normal, oligomenorrhoea, and amenorrhoea. Most patients fell into the oligomenorrhoea category, which comprised 47.5% of patients. 31.2% of patients reported normal menstrual cycle pattern, and 21.2% of patients reported amenorrhoea (Table 2).
Table 1
Descriptive analysis of the age, body mass index, and infertility duration of the included the women
| Age | Infertility duration | BMI | |
|---|---|---|---|
| Mean | 28.39 | 3.214 | 29.675 |
| Median | 28.00 | 3.000 | 30.550 |
| Minimum | 19 | 1.0 | 19.6 |
| Maximum | 38 | 6.0 | 37.0 |
Table 2
Descriptive analysis of the menstrual pattern
| Frequency | Percentage | Valid percent | Cumulative percentage | |
|---|---|---|---|---|
| Normal | 25 | 31.2 | 31.2 | 31.2 |
| Oligo-menorrhoea | 38 | 47.5 | 47.5 | 78.8 |
| Amenorrhoea | 17 | 21.2 | 21.2 | 100.0 |
| Total | 80 | 100.0 | 100.0 | – |
There was a significant positive correlation between the menstrual pattern of patients and their BMI. The higher the BMI, the more the pattern shifted towards abnormal (oligomenorrhoea or amenorrhoea) (Table 3, Fig. 1).
Table 3
Pearson correlation between body mass index and menstrual pattern
| BMI | Menstrual pattern | ||
|---|---|---|---|
| BMI | Pearson correlation | 1 | 0.771** |
| Sig. (2-tailed) | – | 0.000 | |
| N | 80 | 80 | |
| Menstrual pattern | Pearson correlation | 0.771** | 1 |
| Sig. (2-tailed) | 0.000 | – | |
| N | 80 | 80 |
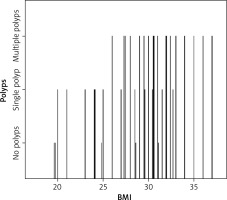
In the study group, the performed office hysteroscopy confirmed the following findings: 62 patients had endometrial polyp(s), 6 patients had a polypoid endometrium, 10 patients had an isolated increase in endometrial thickness, and 2 patients had a small submucosal fibroid (less than 1.5 cm). The majority had multiple polyps – 39 women (62.9%), while the rest had only a single polyp – 23 women (37.1%). The sizes of the found polyps were between 0.6 cm and 1.8 cm. Among the 62 women with polyps, 37 (59.7%) had a BMI of 30 or more. The majority of the multiple-polyp group fell into the category of women with a BMI of 30 or more. Further analysis revealed a statistically significant positive relationship between high BMI and an increased number of polyps (Table 4, Fig. 2).
Discussion
Infertility is a critical condition in women with PCOS. It is caused not only by anovulation but also by endometrial abnormality. However, the use of office hysteroscopy in the diagnostic work-up for infertility in high-risk patients before embarking on costly and invasive in vitro fertilization (IVF) therapy is still controversial. Our study found that a significant percentage of ovulatory PCOS patients also had endometrial polyps – 62 patients out of 81 (76.54%). Also, we noticed an increase in the incidence and number of polyps with patients of greater BMI.
We assessed 2 theories related to the development of endometrial polyps in PCOS patients: obesity and hyperoestrogenism. Because it is well-established in the literature that obesity is associated with both PCOS [8] and endometrial polyps [9], it is also well-established that PCOS patients have a hyperoestrogenic state [10]. Furthermore, oestrogen has a strong association with endometrial proliferation and polyp formation [11, 12].
Obesity
Obesity is a common finding in women with PCOS, and between 40 and 80% of women with this condition are overweight or obese [8]. It is defined as having a BMI greater than or equal to 30 kg/m^2 and further classified into 3 classes [13]:
obesity class I – BMI 30–34.9 kg/m^2,
obesity class II – BMI 35–39.9 kg/m^2,
obesity class III – BMI greater than or equal to 40 kg/m^2 (also referred to as severe, extreme, or massive obesity).
We noticed an increase in the incidence of polyps in patients with higher BMI, and several other studies have affirmed similar findings. In one such study, Önalan et al. evaluated the relationship between age, duration of infertility, oestrogen levels, size and numbers of polyps, and obesity in 223 patients treated for IVF. Their study showed that the size and number of polyps are significantly greater in obese patients than in non-obese patients. Our study found that obesity holds an independent risk factor for the development of endometrial polyps, and there was a positive relationship between BMI and the size of the polyps. Given that obesity is an independent risk factor in developing endometrial polyps, as well as a clinical situation related to polyp size and number, the authors suggested the routine office hysteroscopic evaluation of all infertile patients with a BMI ≥ 30 before treatment with IVF [14].
Oestrogen
The exact cause of endometrial polyps is unknown; however, it is well-documented that endometrial polyps are sensitive to oestrogen [15], a hormone that promotes thickening of the womb lining. Hormonal factors related to oestrogen excess have been implicated in the pathogenesis of endometrial polyps, such as obesity, late menopause, and unopposed oestrogen in either hormone replacement therapy or PCOS [14].
Hyperinsulinaemia is common in PCOS, and it may contribute to high oestrogen levels by enhancing endogenous endometrial oestrogen production, because insulin stimulates aromatase activity in both endometrial glands and stroma [16].
Moreover, free oestrogen and testosterone are elevated in the circulation in the setting of hyperinsulinaemia; also, in part, because of insulin’s downregulation of sex-hormone-binding globulin (SHBG) [17].
Hence, overall, the main effect of insulin on the ovaries is not only increasing androgen production but also deranging the regulation of androgen synthesis so it prevents the downregulation of luteinizing hormone receptors leading to increased production of androgens and oestrone, which, coupled with insulin, lower SHBG, leading to hyperoestrogenism [8].
Based on the results of our study, there seems to be a significant percentage of patients with ovulatory PCOS, who also have endometrial polyps, with a strong correlation between those patients with a higher BMI and the development of polyps. Our results are also backed by the well-established connection between oestrogen and obesity with both PCOS and endometrial polyps.
Another study by Önalan et al. also confirmed the same finding, in which they found that patients with PCOS had a higher number of endometrial polyps. Although further analysis revealed the correlation to be statistically insignificant, this could be due to the small sample size and retrospective study design. When the same study compared patients according to BMI, they found that patients with a BMI of 30 or more had a statistically significantly higher number of endometrial polyps vs. patients with a BMI of less than 30. Additionally, the obesity was positively correlated with the occurrence of polyps, size of the polyps, and the multiplicity of polyp numbers in the correlated analysis [14].
Because very little data appears to be available on this subject, our study may be regarded as a feasibility study. We aimed to enlighten infertility clinicians about the presence of endometrial pathologies (especially polyps) in PCOS patients and to provide an estimate of the incidence of endometrial polyps in patients with PCOS, and identify the need for such patients to be investigated by office hysteroscopy before starting IVF treatment. While further studies on this topic should ideally be performed, we highly recommend investigating PCOS patients with BMI ≥ 30 by using office hysteroscopy to identify any endometrial pathologies that may cause implantation failure before starting IVF treatment.
Conclusions
The results of our study show that there is an association between PCOS and endometrial polyps, and it may be a substantial cause of infertility in such patients. Obesity followed by oligomenorrhoea is the most observed risk factor for having endometrial polyp(s) in PCOS patients. We hope to enlighten infertility clinicians about the presence of endometrial factors (especially polyps) in PCOS patients, and to raise global awareness about the unwanted effects of obesity and advise women of reproductive age to keep an ideal BMI. A large study in the future is needed to confirm our results.