Summary
Myocarditis is a common cause of severe heart failure. One of the treatment modalities to diagnose myocarditis and other heart diseases is left ventricular endomyocardial biopsy, which, although technically feasible and performed in other European countries, is performed extremely rarely in Poland for numerous reasons. The article discusses the technical and periprocedural aspects of biopsy based on our own material, demonstrating step by step each element of the procedure.
Introduction
Apart from the ability to evaluate various cardiac diseases in which noninvasive tests cannot establish an accurate clinical diagnosis, the endomyocardial biopsy (EMB) remains the gold standard for the diagnosis of myocarditis.
The 2007 AHA/ACC/ESC joint guidelines outline 13 clinical scenarios in which EMB should be considered. Of these, 2 are class IB recommendations: (1) newly diagnosed heart failure lasting < 2 weeks associated with normal left ventricular size or enlargement and hemodynamic abnormalities and (2) new heart failure lasting 2 weeks to 3 months associated with left ventricular dilatation and new ventricular arrhythmias, second- or third-degree heart block, or no response to standard pharmacotherapy within 1 to 2 weeks [1]. Worth noting is that although almost 15 years have already passed, these guidelines have not been modified to date. The experts of the European Society of Cardiology in their 2013 position statement on myocardial diseases advocate performing EMB in patients with suspected myocarditis and inflammatory cardiomyopathies, as well as in patients with rapidly progressive heart failure refractory to conventional therapy [2].
In recent years, the utility of EMB has been dramatically enhanced by the use of new research tools, including immunohistochemical and molecular biology techniques, which can accurately determine the etiology of myocarditis. In general, EMB can be used to diagnose and differentiate the etiology of HF or monitor the rejection process in heart transplant patients [3].
EMB can be performed from the right ventricle (RV) or left ventricle (LV). However, since some myocardial diseases involve mainly the LV, the diagnostic utility of RV-EMB is lower than that of left ventricular EMB (LV-EMB) [4]. The Silesian Center for Heart Diseases in Zabrze has had a heart transplant program for over 30 years, in which RV-EMB has been widely used to evaluate the rejection process. In 2016, the first LV-EMB program in Poland was initiated in our institution.
Aim
The purpose of this manuscript is to describe the characteristics of the population that underwent LV-EMB, as well as to address the periprocedural and technical caveats.
Material and methods
Since August 2016, a total of 43 LV-EMBs have been performed in patients with features of left ventricular failure of unclear etiology, including suspected myocarditis. During the same period, 3062 RV-EMBs have been performed in the center. Thus, LV-EMBs represent 1.4% of all biopsies performed.
The indications for LV-EMB were: symptoms of unexplained heart failure with left ventricular ejection fraction (LVEF) < 35% and (1) hemodynamic abnormalities or electrical instability of the heart and/or (2) recent worsening of heart failure (NYHA class II, III, or IV) with no response to standard therapy for 2 weeks. Patients with left ventricular thrombus as well as aortic valve lesions (vegetations or stenosis) and extracardiac vascular pathologies (aortic aneurysm and arterial thrombosis) were excluded from the procedure. In addition, due to high embolic potential, patients with left atrial myxoma were also ineligible for LV-EMB. The clinical data of the studied group are summarized in Table I.
Table I
Baseline characteristics of patients submitted to LV-EMB (n = 43)
[i] LV-EMB – left ventricular endomyocardial biopsy, VF – ventricular fibrillation, VT ventricular tachycardia, LVEDD – left ventricle end-diastolic diameter, LVEDV – left ventricle end-diastolic volume, LVESD – left ventricle end-systolic diameter, LVESV – left ventricle end-systolic volume, LVEF – left ventricular ejection fraction.
Left ventricular endomyocardial biopsy technique
Unlike RV-EMB, LV-EMB is performed from arterial access. The protocol developed at our center adopts femoral access for biopsy. In each patient, significant ischemic heart disease defined as hemodynamically significant coronary artery stenoses ≥ 2.0 mm in diameter was excluded before the LV-EMB [5], either during the coronary angiography performed just before the EMB, or earlier, usually at neighboring centers before the patient was transferred to the clinic.
Before LV-EMB, each patient received 75 mg of acetylsalicylic acid, or a loading dose of 300 mg in aspirin-naïve individuals. Patients were monitored by ECG, invasive blood pressure measurement, and arterial blood oxygen saturation measurement during the procedure. Before the LV-EMB, the international normalized ratio (INR) < 1.5 was required, and anticoagulant therapy had to be discontinued 16 h before and 12 h after the procedure.
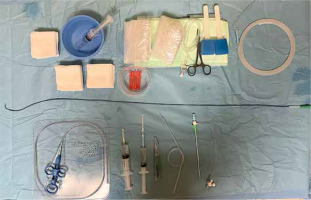
The arterial puncture was performed under local anesthesia in the supine position. The prepared biopsy equipment is shown in Figure 1. After the femoral artery puncture, an arterial sheath, initially 8-French (Fr) and now 7-Fr (Balton, Poland), was introduced. After placement of the arterial sheath, each patient received a bolus of unfractionated heparin (3000–5000 IU) to achieve an activated clotting time (ACT) of 200–250 s. In our study, a total of 16 combined coronary angiographies followed by EMB were performed.
After femoral artery puncture, a 5-Fr pigtail catheter (Boston Scientific, USA) was introduced through a guidewire into the left ventricular lumen via the aortic valve. Then a long J-guide (260 cm, 0.03500) was advanced through the catheter into the LV lumen. The pigtail catheter was removed from the ventricle, and a 90 cm long, 7-Fr MB or JR guiding catheter (Medtronic, USA) was introduced. Subsequently, the J-guide was removed, and a Y-connector was connected to the catheter.
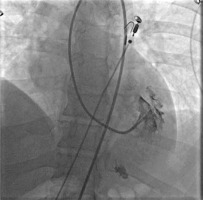
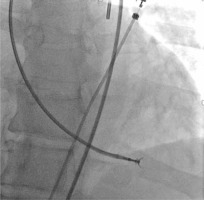
The optimal position and distance between the tip of the guiding catheter and the lateral wall of the LV were checked in the left oblique 20° projection by injecting 5–6 ml of contrast medium. The tip of the catheter should optimally point to the left at the free (lateral) wall of the LV, but it should not touch it (Figure 2). After checking the location of the catheter, 104 cm long Cordis (USA) 5.5 F biopsy forceps were inserted under fluoroscopy guidance near the tip of the guiding catheter. Afterwards, they were opened while still inside the guide catheter and carefully moved toward the lateral wall of the LV (Figure 3). After taking the sample, the closed biopsy forceps were withdrawn slowly into the guide catheter under fluoroscopy control. In total, 6–9 biopsy specimens of 1–2 mm in size with a material volume of approximately 2.4 mm2 were taken from different sites of the LV. To avoid air embolism, before each insertion of the bioptome into the catheter, the forceps were thoroughly rinsed with saline solution.
Figure 3
Collection of left ventricular specimens. Bioptome touches the free (lateral) wall of the left ventricle
Samples for histological and immunohistochemical analysis remained fixed in 4% formalin, and biopsy specimens for the presence of the viral genome were later stored in a dedicated solution (Ambion, Foster City, CA, USA).
After the biopsy, the specimens were collected, the procedure was completed, and the arterial sheath was removed. In all patients, vessel closure devices were used to achieve hemostasis. After the procedure, the patients were monitored for at least 24 h. Immediately after the procedure, echocardiography was performed in each patient to exclude fluid in the pericardial sac. Each patient continued anti-aggregation treatment with acetylsalicylic acid for the next 4 weeks.
As mentioned above, approximately 6–8 ml of contrast was used for each procedure to visualize the position of the guide catheter before the biopsy. The mean fluoroscopy time was 5.4 min, the mean radiation dose was 87 mGy, and the effective dose (DAP) was 748 µGy/m². No post-procedural complications were observed, either local (such as hematomas) or general (stroke, or pericardial tamponade, or fluid).
Discussion
Since the introduction of endomyocardial biopsy in 1962, many improvements have been made in the technique of the collection and analysis of the samples [6]. However, the first comprehensive attempt to evaluate histopathologic biopsy specimens was the 1986 Dallas criteria, for many years the only score for evaluating myocarditis [7, 8].
In the light of new diagnostic modalities (e.g., cardiac magnetic resonance or strain), EMB is not necessary for the diagnosis of myocarditis, or at least its role should have significantly diminished. Unfortunately, even modern noninvasive techniques have their limitations – they do not allow for a precise understanding of the inflammation etiology, which is essential, for example, for the differentiation between acute viral and, e.g., autoreactive myocarditis, which have quite a similar clinical presentation but are treated rather differently. EMB can be used to diagnose heart failure of unknown etiology, cardiac sarcoidosis, amyloidosis, cardiomyopathies, storage diseases, cardiac tumors, and monitoring the effects of anticancer therapy. EMB can also be used to differentiate between constrictive pericarditis and restrictive cardiomyopathy or right ventricular myocarditis and arrhythmogenic right ventricular cardiomyopathy Additionally, LV-EMB is used to confirm the presence of other diseases (such as storage diseases or amyloidosis, or post-anthracycline cardiomyopathy). EMB is also the procedure of choice for monitoring the rejection process in the heart of transplant patients [9].
The old methods of evaluating biopsy specimens for the presence of an inflammatory process were not very specific or sensitive, and additionally, on their basis, it was not possible to predict the prognosis of patients, while the modern immunological diagnostics allow one to make a reliable diagnosis. A recently published retrospective analysis reported the evaluation of 100 consecutive patients who presented to a tertiary center with unexplained cardiomyopathy (ischemic and valvular etiologies were excluded). In these patients, cardiac MRI suggested a diagnosis of myocardial disease in 53% of cases. The combination of cardiac MRI and EMB enabled this number to be increased to 86% [10]. In our previously published material, in the biopsy results, myocarditis was present in 47% of patients, in whom viral myocarditis was confirmed in 21% and chronic reactive myocarditis in 79% [11]. Results of extensive analyses indicate that LV-EMBs provide crucial diagnostic information in 96.3% of cases compared to 71.4% of RV-EMBs, confirming the more profound diagnostic value of LV-EMB [9, 12].
In our center, long (104 cm) 5.5 F diameter bioptomes are used. The same equipment had previously been used in our center for RV-EMBs as well. Nonetheless, other types of bioptomes, such as King’s bioptome or the popular B-18110 flexible bioptome (Medizintechnik Meiners, Germany), with a diameter of 6F and a length of 110 cm, can be used. Their construction differs from the other bioptomes as – instead of a rigid steel spiral – they are built based on a polytetrafluoroethylene tube [4].
Procedural access is essential for both operators and patients. In the biopsy protocol adopted in our center, femoral access is routinely used, as it is comfortable and efficient for operators. Unfortunately, it is less comfortable for the patient and is associated with a longer immobilization time and a higher risk of complications. For this reason, some centers perform biopsies from radial access using sheathless guiding catheters [4, 12–14]. This technique is also safe and will undoubtedly become an extension of the technique used for biopsies in our center in the future.
Unfortunately, the number of EMBs performed in Poland is low. According to the latest published data of the Association of Cardiovascular Interventions of the Polish Cardiac Society, 740 EMBs were performed in Poland in 2014, mostly in patients after heart transplantation [15].
A critical aspect of any invasive procedure is peri- and postoperative complications. In large analyses, the percentage of EMB complications does not exceed 1% [16, 17]. For example, in the largest ever analysis of complications, involving a group of 4221 patients, in which LV-EMB was performed in 84.4% of patients, the complication rate was 0.33%, of which major complications constituted a minority [18]. According to the same analysis, the risk factors of perforation during LV-EMB are the coexistence of: LV free wall thinning (muscle thickness not exceeding 7.5 mm), LV enlargement > 72 mm, and severe LV systolic dysfunction with ejection fraction of about 20% [18]. Moreover, the risk of complications decreases with the number of biopsies performed and when performed by large reference centers [16–18].
In our material, we did not observe any complication, which is explained, on the one hand, by extensive experience in performing RV-EMBs (each of the operators had previously performed >1000 such biopsies) and, on the other hand, by rigorous adherence to the accepted procedural protocol.
The primary limitation to the development of the LV-EMB technique is the lack of reimbursement in Poland for this procedure and examination of the biopsied specimens, as the cost of a comprehensive evaluation of biopsy specimens often exceeds the valuation of the hospitalization by the payer. The second limitation is the need to ship the biopsy specimens to a laboratory abroad, which performs a comprehensive assessment, as a complete histological, immunohistochemical, virological, and genetic evaluation is not possible in Poland.