Introduction
Perimenopause, or the menopausal transition, encompasses that period of time during which physiologic changes mark progression toward a woman’s final menstrual period. This phase begins with the onset of menstrual irregularities and continues until a woman reaches menopause, or one year after amenorrhea has occurred. Perimenopause, then, can last for a variable amount of time, the median of which is four years [1].
The menopausal transition is roughly divided into two phases, which were initially based solely on menstrual patterns. The early transition is characterized by a small increase in the prevalence of common menopausal symptoms, but perturbation of menstrual cycles is minimal, with women having at least one menstrual cycle within the past 3 months. By the time a woman attains the late transition, she has substantial evidence of estrogen deficiency, and the ovarian failure can no longer be compensated for by changes in ovarian and pituitary hormone production. Although some women may be asymptomatic, estrogen deficiency may be accompanied by development of climacteric syndrome (CS) associated with hot flushes, sweating, insomnia, vaginal dryness and discomfort in up to 85% of perimenopausal women [2, 3].
At this age the prevalence of thyroid disease in women reaches its peak. The prevalence of hypothyroidism (HT) among adult women ranges 3–10% and is more common among older women [4].
Hypothyroidism is characterized by elevated serum thyroid-stimulating hormone (TSH) which may be subclinical or overt. In subclinical HT, serum TSH levels are mildly elevated in combination with a normal FT4 level. In the case of overt HT there are markedly elevated TSH levels with a decreased FT4 level. The symptoms of HT are often non-specific and the onset may be insidious.
Thyroid function and the gonadal axes are related throughout the woman’s fertile period. The relationship between the two glands is mutual. Thyroid hormones affect the reproductive function directly and indirectly by such actions as increased synthesis of sex hormone binding globulin, testosterone and androstenedione, reduced clearance of estradiol and androgens, and increased conversion of androgens to estrone. The direct effects are mediated by the presence of receptors for thyroid hormones at the level of oocytes where these hormones act synergistically with follicle stimulating hormone (FSH) (through FSH receptors which are present on granulosa cells).
The main role of estrogens in thyroid physiology is increasing the serum concentrations of thyroxine binding globulin (TBG), a protein synthesized by the liver. The levels of serum TBG change immediately before and soon after menopause; this phenomenon is attributed to increased levels of TBG present in aging and offsetting the lack of estrogen.
Thyroid hormones affect brain function through similar mechanisms to estrogens: cell metabolism, gene expression, inter-cell signal transmission, and modulating the synthesis of enzymes needed for neurotransmitter production. Similarly to estrogens, thyroid hormones modulate the action of noradrenergic, serotoninergic and GABAergic systems in the brain [4].
Great attention is required for the diagnosis of HT in perimenopausal women because such symptoms as weakness, fatigue, weight change, constipation, dry skin, depression, intolerance to cold and memory impairment make it difficult to differentiate between menopausal symptoms and symptoms related to thyroid dysfunction [4–6].
The American College of Physicians has recommended that women older than 50 years with one or more general symptom that could be caused by thyroid disease should be screened with serum TSH testing initially, followed by measurement of FT4 if the TSH level is undetectable or higher than 10 mU/l [5].
Thyroid dysfunction in combination with CS is a comorbid pathology, in relation to the development of metabolic, psychopathological and neurological syndromes in women, the pathogenetic negative impact of which is enhanced by their combination [7].
Timely diagnosis of early climacteric disorders in perimenopausal women is an important medical and social problem of modern medicine. A promising direction in solving this problem at the present level is prognostic mathematical models that will help to predict the development of severe CS.
The aim of the study was to predict the risk of developing CS in perimenopausal women with HT according to the developed algorithm and mathematical model for timely preventive measures.
Material and methods
146 perimenopausal women with autoimmune HT without concomitant chronic somatic diseases with severe or progressive course were examined. The mean age was 46.8 ±0.73 years. The duration of HT was 6.4 ±1.7 years. In order to compare the studied indicators with the variants of the norm, 30 practically healthy women aged 44–52 years (mean age 46.5 ±2.5 years) were examined, selection of whom was carried out taking into account the anamnesis data, and the absence of clinical signs of HT and CS. The research was conducted in compliance with all moral and ethical principles, taking into account the Helsinki Declaration of the World Medical Association for Biomedical Research (World Medical Association Declaration of Helsinki). All patients signed informed consent to participate in the study.
The diagnosis of autoimmune HT was confirmed with an increase in TSH, levels of antibodies to thyroperoxidase and/or antibodies to thyroglobulin and the presence of a characteristic ultrasonographic picture of the thyroid gland.
Assessment of the severity of metabolic, neurovegetative and psychoemotional symptoms was graded according to the Blatt-Kupperman menopause index, which takes into account the most common manifestations of CS and allows one to assess the severity of menopausal disorders. Scoring was performed separately for 3 groups of symptoms; each of the individual symptoms was evaluated depending on the severity on a 4-point scale, then the total value of the Blatt-Kupperman index was assessed: 0–11 points indicates the absence of manifestations of CS; 12–34 points indicates a mild degree of CS; 35–58 points indicates an average degree of CS; and more than 59 points indicates severe CS.
All women were interviewed according to a specially designed questionnaire for predicting the development of severe CS, which included predicted risk factors of CS: ecological living conditions, diet, alcohol consumption, smoking, physical activity, presence of chronic stress and anxiety in the anamnesis, thyroid disease and their gradation from numerical values.
The construction of a prognostic multifactorial mathematical model of CS risk in women with HT was performed using multiple regression analysis. Statistical processing of the results of the study was performed using the statistical package Statistica 10.0. Assessment of the normality of the distribution of traits was performed according to the coefficients of asymmetry and excess, as well as the Shapiro-Wilk and Kolmogorov-Smirnov criteria. Differences between comparison groups at p < 0.05 were considered significant. The quality of the constructed model was assessed by its sensitivity and specificity; the 95% confidence interval (CI) was calculated. To assess the adequacy of the mathematical model for predicting the development of menopausal disorders, indicators of the area under the receiver operating characteristic (ROC) curve (area under curve – AUC) were used, and their 95% CI was calculated.
Results
The examined patients were divided into three clinical groups: group I (n = 43) included women with a level of TSH from 0.4 to 2.0 mmol/ml, which corresponded to the low-normal level of the reference interval for TSH; the average level of TSH was 1.40 ±0.08 mU/l (p < 0.001); group II included 51 women (n = 51) with a level of TSH in the range of 2.1–4.0 mU/l , which corresponded to the high-normal range of reference values; the average level of TSH was 3.55 ±0.09 mU/l (p (0.001); group III consisted of 52 women (n = 52) with HT with a level of TSH above 4.1 mU/l ; the average level of TSH was 7.14 ±0.26 mU/l (p < 0.01).
In 28.8% of the examined patients of perimenopausal age with HT there were no complaints characterizing the manifestations of CS. Accordingly, 71.2% of women had CS of varying severity according to the Blatt-Kupperman menopause index. The structure of the CS was dominated by metabolic and endocrine disorders. Neurovegetative symptoms and psycho-emotional disorders were less common. The severity of individual symptoms of pathological menopause was assessed in points, which were calculated by the Blatt-Kupperman index (Table 1).
Table 1
Symptoms of climacteric syndrome according to the Blatt-Kupperman menopause index in women with hypothyroidism compared with the control group
Assessing the health status in the control group, the mean value of total Blatt-Kupperman index was 10.24 ±1.55 points, indicating no clinical signs of CS, although perimenopausal women had neurovegetative complaints. The results of our studies revealed a close relationship between thyroid dysfunction and menopausal symptoms. In group I, the average value of the total Blatt-Kupperman index was 22.32 ±9.41 points (p < 0.05); complaints of metabolic-endocrine (3.84 ±1.22 points) and neurovegetative disorders predominated (13.24 ±4.73 points, p < 0.05), while psycho-emotional disorders (5.42 ±3.13 points) were mild. In the second group of women, the average value of the total Blatt-Kupperman index was 29.13 ±7.52 points (p < 0.01). Metabolic (4.33 ±2.11 points, p < 0.05) and neurovegetative (19.72 ±6.84 points, p < 0.05) syndromes and moderate psychoemotional disorders (6.14 ±2.43 points) predominated in this group. In the third group of women, the average value of the total Blatt-Kupperman index was 49.01 ±6.31 points (p < 0.05). Climacteric syndrome was manifested by subscales of neurovegetative (21.23 ±5.45 points, p < 0.01), metabolic (8.21 ±4.73 points, p < 0.05) and psychoemotional symptoms (9.34 ±2.83 points, p < 0.05), which were statistically significantly different between the control group and groups I and II.
An important task is the timely interpretation of existing risk factors for menopausal disorders in perimenopausal women with HT in order to correct them to prevent or slow down the development of complications. The introduction of additional indicators in the mathematical model of forecasting, reflecting the individual characteristics of the pathological process, can significantly reduce the errors of diagnosis and prediction of menopausal disorders.
The method of regression analysis was taken as a mathematical model, which allows one to identify the relationship between regression coefficients and values of risk factors that have a likely impact on the development of CS, to identify the relationship between them and predict the likelihood of severe CS.
To build a mathematical model of prediction, the 7 most significant factors were selected that most influenced the risk of developing CS in women of perimenopausal age (Table 2).
Table 2
Risk factors for severe menopausal syndrome and their indexation
To assess the probability of the selected factors, a step-by-step regression analysis was performed: the weight of multicollinear risk factors for CS was determined, and a correlation matrix was constructed with calculation of correlation coefficients and determination of β regression coefficients, which reflect for each factor included in the analysis its relationship to the chances of influencing the development of CS in the surveyed women (Table 3).
Table 3
Predictors with multiple logistic regression coefficients designed to determine the risk of climacteric syndrome in women with hypothyroidism
Risk factors in which the probability level p (value) was > 0.05 were excluded from the analysis. Seven risk factors with the significance level p< 0.05 were included in the mathematical model.
According to our research, the analysis of regression coefficients of the logistic mathematical model shows that a significant predictor of the risk of severe CS was the presence of thyroid disease (β = 0.179) in the anamnesis, including HT, observed in 146 women (82.9%). Significant predictors of the development of CS in women were their low physical activity (β = 0.051), unfavorable environmental living conditions (β = 0.051), the presence of chronic stress in the anamnesis (β = 0.044) and anxiety (β = 0.03), and bad habits: smoking (β = 0.028), alcohol consumption (β = 0.039).
Based on the obtained results of multiple regression analysis of predicting the probability of developing CS (Table 3), a mathematical model of multiple regression was built to determine the risk factor for climacteric syndrome (RFCS):
RFCS = 0.197 + (X1 × 0.028) + (X2 × 0.039) + (X3 × 0.051) + (X4 × 0.051) + (X5 × 0.044) + (X6 × 0.03) + (X7 ×0.179),
where: RFCS is the risk factor for the development of CS in women; 0.197 is a constant; X1–X7 are risk factors with regression coefficients.
To confirm the quality of the developed prognostic value of the mathematical model, we calculated the predicted value of the dependent variable relative risk of CS in women with HT in the period of perimenopause. The value of the risk factor for the development of CS was in the range from 0 to 0.5 and indicated a low risk of CS, from 0.51 to 0.8 indicated a medium risk, and more than 0.81 indicated a high risk of CS. The prognosis of the dependent variable of the risk factor for CS was expected with a high probability in 69 (47.3%) women with HT, with a medium probability in 52 (35.6%) women with HT, and with a low probability in 25 (17.1%) women with HT.
According to the developed mathematical model for predicting the development and progression of CS in women with HT, the predicted value of the risk factor for high-grade CS was determined in 63 (43.2%) women with HT, which confirms the high sensitivity of the prognostic model.
The high accuracy of the mathematical model was also proved in 49 (33.6%) women, in whom the development of CS with a medium degree of risk was predicted.
Low risk of CS was predicted in 24 women (16.4%) with HT.
Correspondence of the forecasted results with the theoretically expected results in the group of low risk of CS was recorded in 96.9%, medium risk of CS in 93.8%, high risk of CS in 90.6% of cases. This confirms the high prognostic accuracy of this mathematical model.
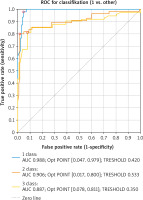
To determine the prognostic value of the mathematical model of forecasting, ROC analysis was performed, ROC curves for degrees I–III of CS risk were obtained, and the corresponding AUC values were determined to assess the quality of the proposed mathematical model (Fig. 1).
As can be seen from Figure 1, area under the curve AUC1 = 0.988 (quality of the classification of the first degree RFCS); AUC2 = 0.906 (quality of classification of the second degree RFCS); AUC3 = 0.887 (quality of classification of the third degree RFCS). Thus, according to the ROC analysis, the prognosis of first and second degrees of risk of climacteric syndrome is distinctive, and for third degree high.
To determine the effectiveness of this model in predicting menopausal disorders in women with HT, we describe a clinical example of calculations:
A 48-year-old patient had a diagnosis of primary HT, subcompensation stage, moderate severity, with the following indicators: the patient does not smoke (X1-0), drinks alcohol no more than twice a week (X2-2), notes moderate physical activity (X4-2), has lived in the city for more than 10 years with adverse environmental conditions (X3-3), notes constant stressful situations (X5-3) and feelings of anxiety (X6-3, works as a teacher), and HT was diagnosed 3 years ago (X7-1).
According to the mathematical formula, by entering the numerical values of the relevant risk factors in the mathematical model of regression analysis, we calculate the RFCS:
RFCS = 0.197 + (2 × 0.039) + (3 × 0.051) + (24 × 0.051) + (3 × 0.044) + (3 × 0.03) + (1 × 0.179) = 0.93; this patient has a high risk of climacteric disorders, as RFCS is 0.93.
Thus, we can conclude that even in the absence of clinical complaints of CS in women with HT, the physician, using a prediction model based on collected anamnestic data, according to the developed questionnaire, can predict the development and progression of climacteric changes. After that, the requirement for additional laboratory tests can be determined and the risk of complications of CS prevented or reduced.
Discussion
According to the developed mathematical model, the most significant predictor of the risk of CS developing was thyroid pathology, in particular HT. This may indicate the impact of menopausal status on thyroid function. Several studies have reported an increase in the level of serum TSH and decreased serum fT4 with age [6, 8] which leads to the rise in frequency of HT in perimenopausal women.
In this study, we observed that a significant risk factor for severe CS was a history of chronic stress and anxiety. In perimenopause, women have a decrease in estradiol and an increase in gonadotropic hormones, the importance of which in the development of CS is beyond doubt [9], which may be accompanied by an increase in depression and anxiety [10, 11] in menopausal women. Some studies confirm the relationship between the presence of a history of chronic stress and severe clinical manifestations of CS [11, 12].
Another important predictor of the risk of CS developing was low level of physical activity in women. Physical activity and maintenance of proper body weight are the main factors in prevention of the development of cardiovascular disease, osteoporosis and diabetes in perimenopausal women [13, 14]. According to the Australian scientists Thomas et al., dosing exercise during menopause improved sleep quality and reduced hot flashes – one of the most common menopausal symptoms in women [15]. Shepherd-Banigan et al. found that regular exercise can be a useful therapy to reduce the manifestations of vasomotor symptoms during menopause [16]. These studies coincide with the results of our observations on the importance of the impact of physical activity in predicting the severity of menopausal disorders in women with HT.
There is still insufficient evidence of the potential impact of environmental factors on menopause. A 20-year European study, which included 1,955 women, of whom 1,224 were menopausal during the study period, found that living in clean areas was associated with a later onset of menopause by 1.4 years [17]. These data confirm the results of our observations on the impact of adverse environmental conditions on prediction of the risk of severe CS. Thus, the state of modern ecology can be considered as an important predictor of the development of premature symptoms of CS.
It is known that in women who smoke, the menstrual cycle is shorter due to the reduction of its follicular phase, and menopause occurs faster by several years. It has also been found that women who smoke have lower levels of anti-Müllerian hormone and inhibin B, which reflect ovarian aging and follicular reserve [18]. An analysis of studies from seven countries (Australia, Denmark, France, Japan, Sweden, the United Kingdom, and the United States) found that the likelihood of early menopause was related to the intensity, duration, and earlier onset of smoking. Smoking duration is a significant predictor of premature and early menopause, and smoking cessation reduces the risk of premature menopause [19]. Excessive use of alcohol is more common among men, but recent statistical analysis showed that an increase in alcohol consumption is also observed among women [20]. Over the past 10 years, alcohol consumption among women in the United States has increased by 84%, and among men by 35% [21]. Alcohol abuse affects reproductive function, menstrual cycle and lifelong hormone levels, including in menopausal women [22]. During perimenopause, estradiol and progesterone levels first fluctuate and then gradually decline to low levels in the postmenopausal period. In addition to hormonal changes associated with menopausal transition, another important factor in alcohol excessive consumption in women is the presence of negative mood, regular stress and depression [23]. The analysis of the literature confirms the results of our linear regression analysis with the selection of the most significant risk factors for the development of CS in women with thyroid dysfunction.
Conclusions
The developed algorithm of examination and mathematical model for predicting the development of CS in perimenopausal women with HT are highly informative and make it possible to determine in advance the contingent of women with a high probability of CS, for active early diagnosis, prevention and treatment tactics among this cohort of patients, which will reduce the risk of CS and its complications. The decay of women’s health starts many years before menopause and prevention of its consequences is an important task for the clinicians.