Summary
Prediction of mortality and major adverse cardiac/cerebral events with the age, creatinine and ejection fraction (ACEF) score has been previously presented in the literature. The predictive value of this scoring for bleeding events has not yet been studied. Our study aimed to investigate the predictive value of the ACEF score for bleeding and mortality events in ST-elevation myocardial infarction patients treated with bail-out tirofiban therapy. Since the ACEF score can be calculated easily and can be applied to routine practice, it would be rational to use it in this patient group.
Introduction
ST-segment-elevation myocardial infarction (MI) (STEMI) patients remain at high risk for major adverse cardiac, cerebral and hemorrhagic events despite optimal medical therapy and successful primary percutaneous coronary intervention (PCI) (pPCI) [1]. According to current guideline recommendations, it is necessary to use dual antiplatelets for at least 1 year in the treatment of acute coronary syndromes (ACS) [2]. Although these medications are known to reduce ischemic events, there is strong evidence that there is a significant relationship between dual antiplatelet use and increased bleeding complications and mortality [3].
In patients with ACS, numerous bleeding risk-stratification scores have been developed with differing advantages or disadvantages [4, 5]. Although the age, creatinine, and ejection fraction (ACEF) score was first developed to predict mortality in elective coronary artery bypass graft (CABG) operations [6], whether it could be a predictor of bleeding events in conservatively treated patients has also been discussed in the literature [7]. Tirofiban is a glycoprotein IIb/IIIa inhibitor and recommended as a bail-out therapy in STEMI patients [8]. A significant decrease in the frequency of adverse cardiac events after the use of tirofiban in addition to heparin has been observed in patients with ACS, while the frequency of bleeding events does not increase [9, 10].
Aim
We conducted the present study based on the hypothesis that the ACEF score would therefore be able to predict Bleeding Academic Research Consortium (BARC) bleeding events of grade 3, 4, or 5 and mortality in STEMI patients undergoing pPCI with bail-out tirofiban therapy (BOTT).
Material and methods
Study design
This was a retrospective observational study conducted at Dr. Siyami Ersek Thoracic and Cardiovascular Centre between January 2012 and December 2016 of patients who underwent pPCI with BOTT following a diagnosis of STEMI. It was approved by the Haydarpasa Numune Education and Research Hospital ethics committee (approval no. 2021/81) and carried out following the principles of the Declaration of Helsinki. Written informed consent was obtained from the patients during their hospitalization.
Study population
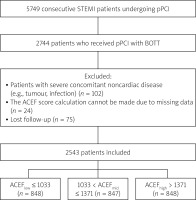
The medical records of 5,749 consecutive STEMI patients undergoing pPCI were scanned and 2,744 patients who received BOTT were selected for inclusion in this study. Patients with a life expectancy of less than 1 year due to severe concomitant noncardiac disease (e.g., tumor, infection), those with missing data for ACEF score calculation, and those lost to follow-up were excluded from the study. Finally, a total of 2,543 patients were enrolled for analysis (Figure 1).
Data collection
Demographic, clinical, laboratory, and angiographic characteristics of study participants were obtained from the hospital database. The left ventricular ejection fraction (LVEF) was calculated according to guideline recommendations by a blinded echocardiographer using the standard biplane Simpson method. pPCI operations were carried out per the guideline recommendations and the types of interventions applied during the procedure were chosen according to the operator’s discretion.
After hospital discharge, all patients were instructed to use dual antiplatelet therapy for at least 1 year and were followed up at outpatient clinics regarding any adverse cardiac or bleeding events after discharge. Any such events they experienced were accessed via the social security institution database and, when necessary, the patients were contacted by phone and additional information about their clinical status was obtained.
Definitions
The ACEF score was calculated according to the following previously defined formula [6]: age/left ventricular ejection fraction + 1 (if creatinine was > 2 mg/dl). Then, patients were stratified into three tertiles based on their ACEF scores.
Anemia was defined using the World Health Organization definition: hemoglobin < 12 g/dl for women and < 13 g/dl for men [11].
BARC bleeding events of grade 3, 4, or 5 (BARC 3-5) during a 30-day period were analyzed according to the following definitions: BARC 3a: overt bleeding plus hemoglobin drop of 3 to < 5 g/dl (provided hemoglobin drop is related to bleeding); transfusion with overt bleeding, BARC 3b: overt bleeding plus hemoglobin drop ≥ 5 g/dl (provided hemoglobin drop is related to bleeding); cardiac tamponade; bleeding requiring surgical intervention for control; bleeding requiring IV vasoactive agents, BARC 3c: intracranial hemorrhage confirmed by autopsy, imaging, or lumbar puncture; intraocular bleed compromising vision, BARC 4: CABG-related bleeding within 48 h, BARC 5a: probable fatal bleeding, BARC 5b: definite fatal bleeding (overt or autopsy or imaging confirmation) [12].
Endpoints
The primary endpoint of this study was BARC 3–5 events or mortality within 30 days after pPCI. The secondary endpoint included long-term all-cause mortality.
Statistical analysis
All analyses were conducted using the program SPSS Statistics version 22.0 (IBM Corporation, Armonk, NY, USA). After deploying the Kolmogorov-Smirnov normality test for data, continuous variables were represented as mean ± standard deviation or median (interquartile range) values. Those variables with normal distribution were tested with a one-way analysis of variance, while if the variables were nonparametric, they were assessed with the Kruskal-Wallis test. Categorical variables were represented as number (percentage) and were assessed using the chi-squared or Fisher’s exact test.
Multinomial logistic regression analysis and Cox proportional hazards modeling were used to compare short- and long-term mortality between the tertiles. Binary logistic regression analysis was performed to evaluate the predictors of bleeding events. Receiver operating characteristic (ROC) curves were constructed to assess the ability of the ACEF score to predict events. The long-term survival was generated by the Kaplan-Meier method, and the log-rank test was used to evaluate differences between the groups.
Results
The study population included 2,543 patients, who were stratified according to the ACEF score tertiles as follows: 848 patients in T1 (ACEFlow ≤ 1.033), 847 patients in T2 (1.033 < ACEFmid ≤ 1.371), and 848 patients in T3 (ACEFhigh > 1.371). Baseline clinical, demographic, and angiographic characteristics of the study participants are listed in Table I. In patients with higher ACEF scores (T3), age and basal creatinine values were already high; additionally, patients in the T3 group were more likely to have diabetes mellitus, hypertension, and a prior history of MI, PCI, and CABG. The highest number of patients presenting with anterior MI was in T3. While multivessel disease was common in the T3 group, most of the patients in the T1 group had single-vessel disease. After the pPCI procedure, Thrombolysis in Myocardial Infarction grade III flow was the lowest in the T3 group.
Table I
Baseline characteristics of patients categorized according to ACEF score tertiles
[i] ACE-I – angiotensin converting enzyme inhibitor, ARB – angiotensin receptor blocker, BMI – body mass index, LVEF – left ventricle ejection fraction, POBA – plain old balloon angioplasty, PTCA – percutaneous transluminal coronary angioplasty, GFR – glomerular filtration rate, RDW – red cell distribution width, MPV – mean platelet volume, ACEF – age – left ventricular ejection fraction and creatinine.
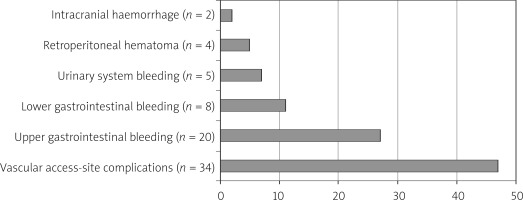
Upon examining the causes of BARC 3–5 events (n = 73), most were the result of femoral vascular access-site complications (n = 34; 47%), while others included upper gastrointestinal bleeding (n = 20; 27%), lower gastrointestinal bleeding (n = 8; 11%), urinary system bleeding (n = 5; 7%), retroperitoneal hematoma (n = 4; 5%) and intracranial hemorrhage (n = 2; 2%) (Figure 2).
The ACEF score significantly predicted the rates of BARC 3–5 events and mortality within 30 days as well as the rate of all-cause mortality at 3 years. In-hospital and 30-day BARC 3–5 events occurred more frequently in T3 patients (1.6%, 2.8%, and 4.1%, respectively). Further, 30-day and 3-year mortality rates were also higher in the T3 group (1.7%, 3.5%, and 7.1% vs 6.4%, 11%, and 19.8%, respectively). Table II lists unadjusted and adjusted multinomial logistic regression and Cox proportional hazard analysis results for BARC 3–5 events and mortality rates. T3 patients were 2.56 times more likely to experience BARC 3–5 events (95% confidence interval (CI): 1.37–4.80) than T1 patients, who were considered as the reference group. Also, the highest 30-day and 3-year mortality rates were found among patients in T3 with 4.53-fold higher 30-day mortality (95% CI: 2.51–8.18) and 3.56-fold higher 3-year mortality (95% CI: 2.58–4.91) rates than patients in T1. These significant relationships persisted even after making adjustments for all confounders.
Table II
Unadjusted and multivariable regression analysis for 30-day BARC 3–5 bleeding events – mortality and 3-year mortality stratified by ACEF score tertiles
| Variable | ACEF (< 1.033) (n = 848) | ACEF (1.033–1.371) (n = 847) | ACEF (> 1.371) (n = 848) |
|---|---|---|---|
| BARC 3–5 bleeding (30-day): | |||
| Number of events | 14 | 24 | 35 |
| Events (%) | 1.6 | 2.8 | 4.1 |
| Event, OR (%95 CI): | |||
| Model 1: unadjusted | 1[Reference] | 1.49 (0.87–2.50) | 2.56 (1.37–4.80) |
| Model 2: adjusted for age and sex | 1[Reference] | 1.21 (0.69–2.13) | 1.49 (0.68–3.29) |
| Model 3: adjusted for comorbidities | 1[Reference] | 1.48 (0.87–2.52) | 2.34 (1.23–4.46) |
| Model 4: adjusted for all covariatesa | 1[Reference] | 1.25 (0.64–2.49) | 1.59 (0.73–3.50) |
| 30-day mortality: | |||
| Number of deaths | 14 | 30 | 60 |
| Mortality (%) | 1.7 | 3.5 | 7.1 |
| Mortality, OR (%95 CI): | |||
| Model 1: unadjusted | 1[Reference] | 2.07 (1.32–3.24) | 4.53 (2.51–8.18) |
| Model 2: adjusted for age and sex | 1[Reference] | 1.74 (1.08–2.8) | 3.06 (1.51–6.2) |
| Model 3: adjusted for comorbidities | 1[Reference] | 2.09 (1.37–3.30) | 4.17 (2.29–7.6) |
| Model 4: adjusted for all covariatesa | 1[Reference] | 1.36 (0.73–2.43) | 2.43 (1.15–5.23) |
| 3-year mortality: | |||
| Number of deaths | 55 | 92 | 168 |
| Mortality (%) | 6.4 | 11 | 19.8 |
| Mortality, HR (%95 CI): | |||
| Model 1: unadjusted | 1[Reference] | 2.02 (1.54–2.66) | 3.56 (2.58–4.91) |
| Model 2: adjusted for age and sex | 1[Reference] | 1.58 (1.18–2.12) | 1.91 (1.28–2.86) |
| Model 3: adjusted for comorbidities | 1[Reference] | 2.01 (1.52–2.66) | 3.50 (2.51–4.87) |
| Model 4: adjusted for all covariatesa | 1[Reference] | 1.69 (1.25–2.27) | 2.51 (1.77–3.51) |
a Includes demographics (age and sex); comorbidities (hypertension, diabetes mellitus, hyperlipidemia, smoking, previous myocardial infarction, previous myocardial infarction, previous percutaneous coronary intervention, previous coronary artery bypass graft surgery), medication at discharge, procedural characteristics (infarct related artery, POBA, direct stenting, PTCA + stenting, thrombus aspiration), admission laboratory values (glucose, creatinine, leukocyte, admission anemia).
To evaluate the parameters that predict BARC 3–5 events across the entire study population, we performed a logistic regression analysis. History of MI, diastolic blood pressure, admission anemia, and ACEF score were deemed to be independent predictors of BARC 3–5 events in the univariate analysis. During the stepwise backward logistic regression analysis, when using a model adjusted for the aforementioned parameters, admission anemia (OR = 1.176, 95% CI: 1.079–2.037; p = 0.043) and ACEF score (OR = 3.903, 95% CI: 2.806–5.326; p < 0.001) were independently associated with BARC 3-5 events (Table III).
Table III
Univariate analysis and multivariate model for prediction of BARC 3-5 bleeding events in the entire study population
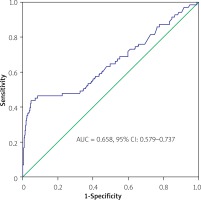
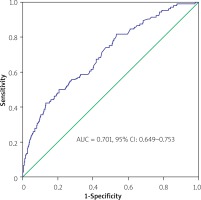
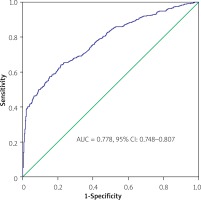
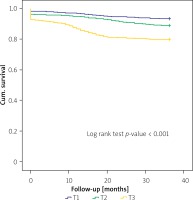
In the ROC analysis, the area under the ROC curve (AUC) values of the ACEF score for 30-day BARC 3–5 events and 30-day and 3-year mortality rates were 0.658 (95% CI: 0.579–0.737), 0.701 (95% CI: 0.649–0.753) and 0.778 (95% CI: 0.748–0.807), respectively (Figures 3–5). Additionally, the 3-year Kaplan-Meier overall survival rates for T1, T2, and T3 were 93.6%, 89%, and 80.2%, respectively (Figure 6).
Discussion
The current study analyzed the prognostic implications of the ACEF score and compared 30-day bleeding and mortality events and 3-year mortality events among STEMI patients undergoing pPCI in conjunction with BOTT. The principal finding was as follows: bleeding and mortality events were more common in patients with higher ACEF score tertiles. Patients with higher ACEF scores were older and more fragile with impaired left ventricular systolic function, so more bleeding and mortality events are to be expected in this population. Among STEMI patients, non-life-threatening minor hemorrhages are common, and rare instances of major hemorrhage can occur. The use of the BARC classification scheme seems rational in these patients [13].
Since the risk of recurrent ischemic events after PCI is higher in patients with an ACS diagnosis, a period of at least 12 months of dual antiplatelet therapy is required. During invasive procedures, some patients may develop iatrogenic coronary artery dissection, angiographic no-reflow phenomenon, or distal embolization, and these patients may need to be given bail-out glycoprotein IIb/IIIa inhibitors along with unfractionated heparin therapy [14]. With the concomitant use of these medications, an increase in the frequency of bleeding events can be seen in the hospital period [15]. Although clinical studies [16–21] have reported that tirofiban therapy may facilitate minor bleeding, it has been reported that no significant increase in major bleeding events occurs even when this drug is used concomitantly with other medications. Since we specifically included STEMI patients treated with tirofiban in our study, we could not predict whether the ACEF score can predict bleeding events as compared to patients without tirofiban therapy.
Current guidelines recommend the use of bleeding risk scores in patients with ACS to predict in-hospital bleeding events [22]. Vascular access site complications are the most common cause of in-hospital bleeding. However, gastrointestinal system, urinary, and, rarely, intracranial bleeding may occur [23]. Bleeding risk scores are specifically designed to determine the in-hospital risk level, so they may be insufficient to predict long-term bleeding events [24]. Following the existing literature, we observed that bleeding most frequently resulted from vascular access-site complications and we wanted to investigate only those bleeding events in the first 30 days instead of in the long term.
Multiple risk-prediction models have been proposed for predicting post-MI bleeding and mortality outcomes, including clinical, angiographic, or combined scoring systems [25–27]. The ACEF score was first developed to predict the endpoints of patients undergoing bypass surgery [6]. Afterward, different models were created for patients with ACS by combining the ACEF score with various clinical and angiographic variables, which enhanced the power of this scoring scheme to predict outcomes [28–31]. On the other hand, in a recent study by Kristić et al. with NSTEMI patients, the ACEF score showed better discrimination than the other six clinical, angiographic, or combined risk scores [32].
In our study population, the prevalence of anemia at admission was significantly higher in those with high ACEF scores. Anemia has an established adverse prognostic value in the setting of ACS [33]. Vrsalovic et al. found that the presence of anemia at admission is an independent predictor of 30-day mortality in STEMI patients [34]. When we investigated the BARC 3–5 event development by including the entire patient population, we also found that admission anemia and ACEF score were independent predictors. Moghaddam et al. did not observe a significant increase in all-cause mortality in STEMI patients with anemia at admission but found a significant relationship with major bleeding [35].
However, although the predictive power of the ACEF and modified ACEF scores in STEMI patients has been proven, no clinical study in the literature has yet evaluated both bleeding and mortality events in patients undergoing pPCI with concomitant BOTT. Based on the main findings of our study, we concluded that the ACEF score, which is a practical and easily calculable score, in addition to predicting mortality, can also be a powerful predictor of bleeding events, so we think that our study contributes to the literature in this respect.
This was a retrospective observational study conducted at a single center and, as such, has the inherent limitations of this kind of study design. In the study population, we did not adopt bleeding risk-scoring systems that included clinic and angiographic variables. Addition of these scales to the ACEF score may have resulted in a clinically meaningful improvement in the prediction of bleeding events. Also, we were only able to analyze BARC 3–5 events within 30 days in this study, and our analysis of bleeding events occurring within the 3-year follow-up period may further contribute to the literature.
Conclusions
Bleeding and mortality events are more common among patients with higher ACEF scores. The ACEF score, which can be calculated easily and has significant power for predicting clinical events, seems rational to apply to STEMI patients in whom tirofiban is administered together with other drugs.