Introduction
Diagnostic video-assisted thoracoscopic (VATS) lung wedge resections are commonly performed in various indications, such as solitary pulmonary nodules, interstitial lung diseases, or pulmonary lesions with suspected metastasis. Pulmonary wedge resection by VATS performed by Miller in 10 cases in the modern sense was reported in 1992. Its reliability has been demonstrated in larger series and later became a standard procedure for wedge resections [1, 2]. Usually, the chest tube and underwater seal drainage system are applied in patients undergoing lung resection, and the tube is removed according to air leak and/or drainage. While the known history of tube thoracostomy dates to ancient times, the underwater seal chest drainage system was defined for empyema at the end of the 1800s [3]. The acceptance of tube thoracostomy as a standard procedure in treating pneumothorax and hemothorax dates to the late 1950s [4]. Pain due to the chest tube can occur both nociceptively by skin irritation and neuropathically by intercostal nerve compression or injury. In addition, acute pain may occur, associated with the patient’s movement in the postoperative period [5]. Another type of chest tube-related pain emerges when removing the chest tube. As a result, chest tube-induced pain, which may affect the patient’s compliance with treatment, encourages surgeons to perform drainless surgery [6]. Drainless VATS, previously applied for sympathectomy and other mediastinal interventions, has gradually progressed to pulmonary resections. According to the literature, the drainless VATS pulmonary wedge resection procedure was started at the beginning of the millennium [7]. The authors reported the advantages of this procedure in terms of postoperative pain and length of hospital stay and emphasized its superiority [8].
Aim
Here, we aimed to investigate the advantages of the drainless VATS pulmonary wedge resection procedure and present the initial experience of our department.
Material and methods
Technique
The patients are intubated with a double-lumen tube and placed in the lateral decubitus position. A 3 cm utility incision is made at the 5th anterior intercostal space. A second 5 mm incision is made at the junction of posterior axillary line and seventh intercostal space for a 5 mm 30-degree optic. After intrathoracic exploration, wedge resection is performed to the targeted area with a 45 mm/4.8 endoscopic linear cutter stapler. The chest cavity is filled with saline solution, lung hyperinflation is performed, and parenchymal air leakage is controlled. When it is confirmed that there is no air leak, the 5 mm optic is moved into the utility incision, the green Nelaton catheter (14 Ch) is inserted into the thoracic cavity from the camera port, and the distal end of the catheter is submerged into culture container filled with saline for providing underwater drainage for providing underwater drainage. The lung is hyperinflated again by the anesthesiologist, and the surgeon checks whether there is an air leak in the underwater drainage. Generally, the stapler line is supported by fibrin sealant application. The utility incision is closed, and the Nelaton catheter is aspirated and removed after confirming that there is no air leak from the anesthesia monitor (Figures 1 A–D). Patients were routinely transferred to the recovery unit after the surgery, and intravenous paracetamol and tramadol infusion were applied for postoperative pain. After ensuring the patients’ orientation and compilation they are transferred to the hospital ward service, and the VAS score was measured by the service nurses at the 2nd, 4th, 6th, 12th, and 24th h and recorded in the patient’s file. The presence of pleural complications such as pneumothorax, subcutaneous emphysema and pleural effusion are evaluated by chest radiographs taken at the end of surgery, at the postoperative 6th and 24th h.
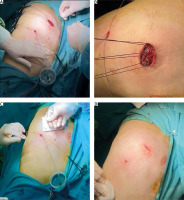
Figure 1
A – A 14-ch green Nelaton catheter is inserted via a camera port and immersed in a water container. B – The utility incision is closed with interrupted polyglactin sutures. C – After the parenchymal leak test is performed, the lung is hyperinflated by the anesthesiologist, while the utility incision is compressed with gauze to prevent subcutaneous emphysema. D – When the air leakage in the container stops, the Nelaton catheter is removed by applying suction
Patient selection for inclusion in the study
After the approval of the local ethics committee, we reviewed the data of patients who underwent VATS-pulmonary wedge resection for various indications between December 2022 and May 2023 retrospectively. Patients whose surgery notes and follow-up records could not be accessed, those who underwent volume reduction surgery, those who had inflammatory conditions such as aspergilloma-tuberculosis (as it would adversely affect the hospitalization process), those who had undergone three or more wedge resections, those who had undergone wedge resection performed with a non-staple method, such as an energy device or electro-cautery, etc., those with severe pleural brits and lymph node dissection, and those with intercostal approximation stitches (it may affects the pain score) were not included in the study. Data of patients collected were age, sex, indications, number of wedge resections, postoperative complications (pneumothorax and subcutaneous emphysema, pleural effusion, etc.), postoperative pain score (visual analog scale, VAS, 1 to 10) and length of hospital stay (days).
Statistical analysis
All analyses were made with the SPSS version 25.0 software. The distribution normality of numerical data was investigated by histogram and Kolmogorov-Smirnov methods. Normally distributed data were given as mean with standard deviation, nonparametric data as median with range-minimum/maximum. Categorical data were given as n and %. The existence of a correlation between categorical groups was investigated using the Pearson χ2 or Fisher’s exact test methods according to sample size. The difference between groups, including numerical data, was investigated with the Student-t test or Mann-Whitney U test according to the normality of distribution. Analyses were performed with a 95% confidence interval, and a p-value less than 0.05 was considered statistically significant.
Results
A total of 35 patients who met the inclusion criteria were included in the study. There were 19 (54.2%) females. Drainless and with-drain groups included 14 (40%) and 21 (60%) patients, respectively. The median age was 59 (range: 20–81), and the median length of hospital stay was 2 (range: 1–4). The mean VAS was 3.2 (SD: 0.6). The most frequent surgical indication was pulmonary nodule in 19 (54.2%) patients, followed by interstitial lung disease (ILD) in 10 (28.5%) patients. The characteristics of patients are given in the Table I. When the patients were divided into two groups, the drainless and with-drain group, the postoperative VAS score and length of hospital stay were significantly low in the drainless group (p < 0.001, Figures 2, 3). There was no significant correlation between the groups in terms of pneumothorax (p = 0.7), subcutaneous emphysema (p = 0.5), age (p = 0.5), gender (p = 0.6), and number of wedge resections (p = 0.9).
Figure 2
The length of hospital stay was significantly lower in the drainless group than in the with-drain group
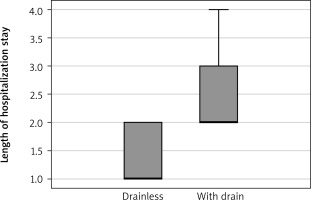
Figure 3
The mean score of the visual analog scale was significantly lower in the drainless group than in the with-drain group
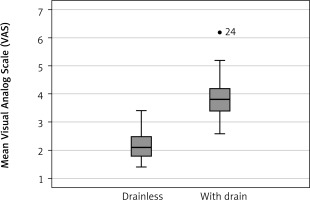
Table I
Characteristics of patients included in the study and results of comparative analysis (n = 35)
| Parameter | Drainless | With drain | P-value | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Categoric variables: | ||||||
| Gender: | ||||||
| Female | 8 | 57.1 | 11 | 52.4 | 0.9 | |
| Male | 6 | 42.9 | 10 | 47.6 | ||
| Indication: | ||||||
| ILD | 4 | 28.6 | 6 | 28.6 | ||
| SPN | 8 | 57.1 | 11 | 52.4 | 0.7 | |
| Pneumothorax | 2 | 14.3 | 4 | 19 | ||
| Number of samples: | ||||||
| 1 | 13 | 92.9 | 19 | 90.5 | 0.8 | |
| 2 | 1 | 7.1 | 2 | 9.5 | ||
| Postoperative pneumothorax: | ||||||
| Yes | 2 | 14.3 | 3 | 14.3 | 0.9 | |
| No | 12 | 85.7 | 18 | 85.7 | ||
| Postoperative SE: | ||||||
| Yes | 4 | 28.6 | 4 | 19 | 0.6 | |
| No | 10 | 71.4 | 17 | 81 | ||
| Numeric variables: | ||||||
| Age [years] | Median with range | 53.5 (20–73) | 59 (22–81) | 0.5 | ||
| Length of stay [days] | Median with range | 1 (1–3) | 2 (2–4) | < 0.0001 | ||
| VAS* (score 1–10) | Mean ± SD | 2.2 ±0.3 | 3.8 ±0.4 | < 0.0001 | ||
Discussion
We aimed to demonstrate the advantages of the drainless VATS pulmonary wedge resection procedure and its preliminary results in this study. The first drainless VATS wedge resection results were published at the beginning of the millennium in the literature [7]. The main factors that lead thoracic surgeons to drainless-VATS surgery are tube-related pain and length of hospital stay. Alanwar et al. reported that in a randomized controlled trial including 24 cases of patients who underwent drainless VATS wedge resection, postoperative pain and length of hospital stay were significantly low in the drainless group [9]. Lesser et al. confirmed the superiority of the drainless VATS-resection group in terms of postoperative analgesic requirement and length of stay in another prospective randomized study [10]. Liao et al. found that pain scores and length of hospital stay were significantly lower in the drainless group in their study, which included 50 patients who underwent uniportal VATS pulmonary wedge resection in each arm [11]. In our study, we used a biportal technique, which includes a utility incision of approximately 3 cm and a second incision of 5 mm for the camera port. We think; fluid, blood, and air can be aspirated more easily with a Nelaton catheter inserted through the camera port, and it is less risky for postoperative pneumothorax than the uniportal technique. We found that the VAS score was significantly lower and the length of hospital stay was significantly shorter in the drainless group, and this result was compatible with the literature.
Patient selection is important for drainless pulmonary resection. According to the literature, patients with emphysematous lungs are unsuitable for this procedure. Yong et al. reported some contraindication criteria for drainless pulmonary resections, such as chronic obstructive pulmonary diseases (COPD), inflammatory lung diseases, history of ipsilateral thoracic surgery, and low FEV1 (< 70%) value [12]. Watanabe et al. stated that air leak detected during the surgery was a contraindication for drainless VATS wedge resection and that patients with bullous-emphysematous lung diseases, pleural effusion, or pleural adhesions were not suitable for this procedure [7]. In another study, in addition to the previous ones, the authors reported that pulmonary lesions larger than 3 cm and more than one wedge resection are exclusion criteria [10]. We performed two wedge resections in 1 patient in the drainless group and two in the with-drain group. We decided on the drainless procedure based on the emphysematous tissue characteristic of the lung and the intraoperative air leak rather than the nodule size or the number of wedge resections. Additionally, we detected that drainless VATS wedge resections could be performed safely in patients with interstitial lung diseases.
Previous studies reported that drainage of air and fluid during the surgery was performed by various drain types, which were removed at the end of the procedure. Yong et al. preferred the Jackson-Pratt drain for this [12]. Lesser et al. used a 24F chest tube and underwater seal drainage system [10]. Li et al. inserted a central venous catheter via the intercostal region at another site from the utility incision and applied -8 cm H2O negative pressure to the catheter [13]. Liao et al. checked the air leak with saline filling to the thoracic cavity and used a 7 mm-silicon CMW drain with a vacuum ball. Then, they decided on the drainless procedure according to the continuation of the collapse of the ball [11]. As described in the methods section, we checked the air leak in three stages. First, we determined whether air leaked from the parenchymal stapler line by saline filling in the thoracic cavity after the lung inflation by the anesthesiologist. Second, we checked for air leak from the Nelaton catheter. Finally, we removed the Nelaton catheter with negative aspiration after confirming no air leak from the anesthesia monitor. This method has some advantages, such as its smaller diameter compared to the classical chest tube and greater cost-effectiveness compared to the central venous catheter and Jackson-Pratt drain.
Since the discrimination of residual air and pneumothorax could not be made clear, the pneumothorax rate is reported as wide in the drainless VATS in the literature. Alanwar et al., Laven et al. and Liu et al. reported the pneumothorax rate in their series as 5.1%, 8.3% and 18.9%, respectively [9, 14, 15]. However, Holbek et al. reported the rate of pneumothorax as 59% in their study, including 51 cases; none of the patients needed tube thoracostomy [16]. Lin et al. stated that pneumothorax requiring tube thoracostomy was correlated with pleural adhesion in their series including 34 cases [17]. In our study, pneumothorax was detected in 2 patients in the drainless group, and it decreased and completely resolved in follow-up radiographs. Since those patients were asymptomatic and pneumothorax completely resolved radiologically the day after surgery, we think this was residual air rather than pneumothorax. Subcutaneous emphysema is one of the unpleasant complications of drainless VATS. Liao et al. reported the rate of subcutaneous emphysema in drainless and with-drain groups as 11% and 6%, respectively [11]. While Li et al. found the rate of subcutaneous emphysema to be 27.2%, Liu et al. reported a very low rate of 3.3%. Additionally, they could not find any cause or predisposing factors for this complication [13, 15]. In our series, minimal subcutaneous emphysema around the utility incision, which was detected radiologically and had no clinical significance, occurred in 4 patients, and none of them required additional intervention. This rate is partially compatible with the literature. The cause of subcutaneous emphysema may be an air transition via the utility incision into thoracic soft tissues during lung hyperinflation by an anesthesiologist. We observed that subcutaneous emphysema did not occur in our last 10 cases with pad compression applied on the utility incision during lung hyperinflation period (Figure 1 C).
Our preliminary study has some limitations. It is a single-centered and retrospective study with a small number of cases. The assignment of patients to arms in a new prospective randomized trial is very difficult. Unexpected intraoperative pleural adhesions or emphysematous lung parenchyma may cause the patient to shift to the with-drain arm and lead to selection bias. In addition, inserting a chest tube into a patient who has no intraoperative air leak may cause more postoperative pain and ethical concerns. Therefore, the decision of chest tube placement in patients undergoing VATS pulmonary wedge resection should be made depending on whether an air leak is detected intraoperatively or there is probable air leak due to pleural adhesions and/or emphysematous lung.






