Introduction
The incidence of gastric cancer (GC) has been declining steadily over the past 70 years [1]; however, GC remains the fourth most common malignancy and the third leading cause of cancer-related mortality worldwide [2, 3], with a million cases diagnosed annually. GC is often either asymptomatic or causes non-specific symptoms only. By the time symptoms appear, the cancer has often reached an advanced stage and may have metastasized. Despite enormous progress in medical technology, the outcome of patients with GC remains poor [4]. Therefore, early detection and treatment of GC is necessary to improve the prognosis of GC. Early gastric cancer (EGC), as defined by the Japanese Society of Gastroenterological Endoscopy in 1962, is invasive GC that invades no more deeply than the submucosa, irrespective of lymph node metastasis (LNM), and it denotes early lesion features of the disease. Today, the superior prognosis of EGC has led to the development of better screening protocols in high risk populations [5–7] and better surveillance guidelines for gastric premalignant lesions.
Recently, endoscopic submucosal dissection (ESD) has revolutionized the treatment of EGC, with the rates of post-therapeutic morbidities and mortalities, hospital stays and financial burden lower than with surgery [8–11]. Moreover, ESD should be applicable to GC patients when following criteria including highly differentiated pathology, clinically node negative status, lesions involving the mucosa, non-ulcerated lesions, and lesion size of less than or equal to 2 cm. However, these criteria are established after postoperative pathology analysis of a large sample of EGC patients. Hence, it is urgent to determine whether the absolute indications of ESD can be identified by non-invasive techniques such as imaging.
Aim
In the present study, we analyzed the postoperative pathological features of patients with EGC who received surgical treatment in Ruijin Hospital from 2012 to 2016, and verified the diagnostic value of ESD indications in LNM. Meanwhile, to determine whether preoperative imaging methods can accurately predict the indications of ESD, ESD indications measured by ES, EUS, and MDCT were compared with the results of postoperative pathological diagnosis.
Material and methods
The study was approved by the institutional Ethics Committee of Ruijin Hospital and followed the principles of the Declaration of Helsinki. Written informed consent was obtained from each participant.
Segment I
Patients
To investigate whether absolute indication criteria (such as tumor size, depth of invasion, ulceration, and differentiation) for ESD are enough to preoperatively determine the lymph node status of EGC patients, a total of 794 patients with postoperative pathology-proven EGC in our organization between 2012 and 2016 were enrolled in this study. Moreover, clinicopathologic characteristics of all patients, such as age, sex, tumor size, differentiation grade, T stage, the presence or absence of ulceration, the presence or absence of lymphovascular/neural involvement, and HER-2 expression were obtained and are summarized in Table I.
Table I
The association between LNM and other clinicopathological factors in EGC (N = 794)
Inclusion and exclusion criteria
The patients were selected based on the following inclusion criteria: (1) no age or sex bias, (2) patients with GC who underwent standard gastrectomy with D2 lymphadenectomy in Ruijin Hospital between 2012 and 2016, (3) postoperative pathology confirmed EGC (pT1 disease). Exclusion criteria were: (1) the presence of synchronous lesions where one or more were T2 or higher, (2) gastrectomy performed after ESD where no tumor residue was found, (3) patients with biopsy proven GC before surgery but no tumor cells identified in postoperative pathology.
Procedure
Based on the data collected from patients, the relationship between the clinicopathological features of EGC and lymph node metastasis was investigated. Briefly, the probability of LNM was studied by age, gender, tumor size, differentiation grade, depth of tumor invasion, presence or absence of ulceration, lymphatic/vascular/nerve invasion, and HER-2 expression.
Segment II
Patients
To determine whether the combined use of GS, EUS, and MDCT could help identify EGC patients meeting the absolute indications for ESD, 86 patients with EGC confirmed by pathological biopsy and suspected by gastroscopy in our organization between January and December, 2017, were included in the study. All participants were preoperatively staged using GS, EUS and MDCT. The absolute indications for ESD for all patients, including tumor size, depth of invasion, ulceration, and differentiation were collected.
Inclusion criteria
Inclusion criteria were: (1) no age or sex bias, (2) patients with biopsy proven GC, suspected to be EGC in initial gastroscopy, (3) diagnostic gastroscopy performed at the digestive endoscopy center of Ruijin Hospital from January to December 2017, (4) patients who have undergone EUS and MDCT for preoperative staging after admission to the department of Gastrointestinal Surgery, ward III, (5) patients who had undergone standard gastrectomy with D2 lymphadenectomy as the definitive therapeutic intervention.
Procedure
After collection of relevant data for the 86 selected patients, preoperative imaging information acquired from MDCT and EUS was separately compared to subsequent postoperative pathology. The procedure was as follows: (1) diagnosis of tumor depth, i.e. the capacity of EUS and MDCT to differentiate T1a and T1b disease was compared to postoperative pathology, (2) diagnosis of node negative status, i.e. the capacity of EUS and MDCT to differentiate clinically node positive (cN+) and clinically node negative (cN-) status was compared to postoperative pathology, (3) identification of the presence or absence of ulcerative findings in gastroscopy was compared to that of postoperative pathology, (4) endoscopic measurements of the lesion size were compared to that of postoperative pathology. The ability of gastroscopy to identify lesions of different sizes, with 2 cm as the cut-off indication for endoscopic treatment, was compared to that of postoperative pathology, (5) accuracy of preoperative imaging in determining the absolute indication criteria for endoscopic treatment was investigated by comparing combined preoperative information with postoperative pathology.
Ethics approval and consent to participate
This study was approved by Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Statistical analysis
All data processing was done using SPSS 21.0 software, and measurement data were expressed as mean ± SD. The χ2 test and Fisher’s exact probability test were used to compare the differences in rates of LNM in different subgroups. P < 0.05 indicates statistical significance. Multivariate logistic regression analysis was performed to identify independent risk factors for LNM in EGC. Receiver operating characteristic (ROC) was used to analyze the accuracy of the combined use of GS, EUS and MDCT in predicting absolute indication criteria for ESD.
Results
Segment I
Relationship of LNM with other clinicopathological status in patients with EGC
As shown in Table II, 794 patients were divided into two groups: the LNM+ group and the LNM– group. The results showed that LNM was significantly associated with ulceration (p = 0.0019), lesion size (p = 0.0018), invasive depth (p < 0.0001), differentiation (p < 0.0001), lymphatic invasion (p = 0.0038), vascular invasion (p < 0.0001), neural invasion (p = 0.01) and HER-2 expression (p = 0.003). However, no significant differences were found between LNM and other clinical parameters, such as age, sex, and tumor location (all, p > 0.05).
Table II
Multivariate logistic regression analysis of independent risk factors for LNM in EGC (N = 794)
Moreover, multivariate logistic regression analysis of independent risk factors for LNM revealed that tumor diameter (p = 0.0071), pT (p < 0.0001), differentiation (p = 0.0001), and vessel invasion (p = 0.0007) were independent risk factors for LNM in patients with EGC.
Diagnostic value of the absolute indications of ESD for LNM
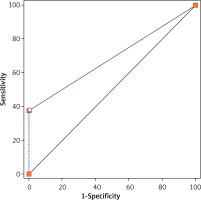
Next, we assessed the potential value of absolute indications of ESD for clinical diagnosis of LNM in 794 patients with EGC. The results showed that ESD indications could effectively distinguish EGC patients with LNM from those with no LNM with an AUC of 0.69 (p < 0.05) (Figure 1; Table III).
Segment II
General clinicopathological features of the subjects
As illustrated in Table IV, there were 54 male and 32 female patients with ages ranging from 33 to 83 years old. During preoperative workup, 44 patients were identified as having nodal involvement (EUS and CT combined), 38 patients as having a tumor size of 2 cm or smaller, 20 patients were diagnosed with mucous disease (T1a, by EUS), and 20 patients had ulcerative lesions on gastroscopy. Tumor location was at the upper third, middle third and lower third of the stomach for 8, 36, and 42 patients, respectively. During examination of postoperative pathology specimens, 24 patients had lymph node metastasis, 52 patients had a lesion sized 2 cm or smaller, 44 patients had T1a disease and 30 patients had lesions with ulcerative findings.
Table IV
Clinicopathological characteristics of preoperative imaging and post-operative pathology
Diagnostic utility values of EUS, MDCT, or EUS + MDCT for tumor depth
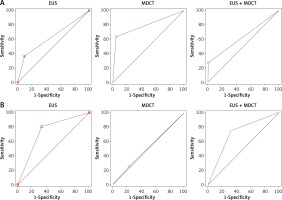
With postoperative pathology as the standard of reference, the diagnostic accuracy values of EUS, MDCT and EUS + MDCT in clinical diagnosis of tumor depth at T1a given by AUC of ROC were 0.63 (sensitivity: 36.36%; specificity: 90.48%), 0.79 (sensitivity: 63.64%; specificity: 95.24%) and 0.64 (sensitivity: 27.27%; specificity: 100%) (Figure 2 A), respectively.
Diagnostic utility values of EUS, MDCT, or EUS + MDCT for LNM
The AUC values of EUS, MDCT and EUS + MDCT in clinical diagnosis of LNM at N stage were 0.73 (sensitivity: 57.14%; specificity: 86.20%), 0.51 (sensitivity: 25%; specificity: 77.42%) and 0.71 (sensitivity: 75%; specificity: 67.74%) (Figure 2 B), respectively.
Diagnostic utility values of GS for tumor size
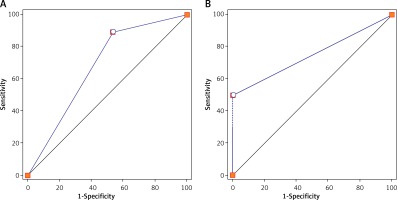
Additionally, with the GS, the AUC values of the ulcerative findings and tumor size were 0.68 (sensitivity: 89.29%; specificity: 46.67%; Figure 3 A) and 0.72 (sensitivity: 61.54%; specificity: 82.35%), respectively. More importantly, the correlation coefficient of the tumor size between endoscopic measurements (tumor diameter: 2.81 ±0.23) and postoperative specimen measurements (2.71 ±0.22) was 0.98, indicating that gastroscopy could accurately reflect the pathological size of the tumor.
Combined diagnostic value of imaging for absolute indication criteria
Based on the postoperative pathology results, the diagnostic accuracy of using all imaging techniques for the preoperative assessment of ESD indications was investigated. As illustrated in Figure 3 B, the AUC was 0.71 with a sensitivity of 42.86% and a specificity of 100%. This suggests that the combined use of GS, EUS and MDCT has a high specificity for selecting suitable candidates for ESD treatment.
Discussion
During the past few years, an increasing number of studies have been able to predict trends of LNM in EGC on the basis of endoscopic findings and clinicopathological features of primary lesions. For instance, Kwee and Kwee [10] identified location of the tumor in the middle stomach, larger tumor size, depressed tumor type, ulceration, diffuse histologic type, and lymphatic tumor invasion as risk factors for LN metastasis in EGC. Kurihara et al. [12] reported that tumor diameter, lymphatic invasion, and depth of invasion were associated with LNM. Moreover, a previous study reported that the rates of metastasis in patients fulfilling absolute and expanded criteria for ESD were found to be low, at 0.3% and 0.4%, respectively [13]. In accordance with previous studies, our data revealed that clinical features, including tumor size above 2 cm, poorly differentiated pathology, tumor depth invasion below the mucosa, and vascular invasion are associated with higher risks of locoregional lymph node involvement. Moreover, none of the 84 cases fulfilling the absolute criteria of ESD were found to have LNM and ESD indications could effectively distinguish EGC patients with LNM from those with no LNM. Taken together, these data indicate that absolute criteria of ESD for EGC are safe and effective for screening patients with LNM.
Given that currently the rates of post-therapeutic morbidity and mortality, hospital stays, and financial burden are lower than for surgery [14–16], the indications of ESD have been accepted by an increasing number of surgeons [17]. However, due to the inaccuracy of preoperative evaluation, patients with EGC are usually treated with standard surgery again after ESD, which will increase the risk of surgery for patients [18]. Hence, an accurate preoperative identification of absolute indication criteria for ESD is of great importance. With the rapid development of endoscopic technology and accumulation of ESD data, a new guideline of ESD indication based on endoscopic imaging will lead to more accurate patient selection for ESD [19]. As a crucial factor to predict LNM in patients with EGC, tumor size measured by GS has been found to be lower than that of postoperative pathological measurements [20]; however, the difference in tumor size between preoperative and postoperative measurements was small, at less than 0.4 cm in approximately 80% of patients. In the present study, our data suggest that GS could distinguish EGC patients with a tumor diameter ≤ 2 cm from those with a tumor diameter > 2 cm with an AUC of 0.72. Moreover, a strong correlation was observed between endoscopic measurements of tumor size and measurements of the postoperative specimen.
The degree of GC ulcer mainly depends on the diameter and depth of the lesion, but endoscopic diagnosis of GC ulcer formation is difficult [19, 21]. Usually, endoscopic examination leads to a high estimation of ulceration, which leads to patients fulfilling the absolute criteria of ESD to miss their chance of treatment. In the present study, endoscopic diagnosis of ulcerative lesions was shown to have a sensitivity and specificity of 89.29% and 46.67%, respectively, with an AUC of 0.68. EUS has been regarded as one of the most valuable methods for detection of the infiltration depth of EGC, with an accuracy of 60–90% for T stage [22–24]. Moreover, MDCT in the detection of T stage and N stage of GC was 77–89% and 69–92%, but its accuracy in the diagnosis of EGC was low [25]. In our study, the results showed that the accuracy of MDCT diagnosis for EGC infiltration depth at T1a stage was superior to that of EUS or EUS + MDCT. On the other hand, in comparison to MDCT or EUS + MDCT, the accuracy of EUS for measuring EGC lymph node involvement at N stage was higher. Actually, both Hasegawa and Aoyagi et al. [26, 27] showed that highly differentiated non-ulcerative cases could present with LNM and therefore preoperative diagnosis of lymph node involvement becomes particularly important. Finally, preoperative assessments of ESD indications from all imaging information were compared with postoperative pathology, and the results of sensitivity and specificity were 42.86% and 100%, respectively.
Conclusions
Our data showed that the absolute indications of EGC were safe with no risks of LNM for patients. Moreover, based on the combined use of GS, EUS and MDCT, a high specificity of patient selection for ESD treatment can be achieved. Nevertheless, our study has several limitations. Firstly, the sample size was small, as only 86 patients fulfilled the inclusion criteria for selection with imaging information. Secondly, imaging information was incomplete. Hence, further comprehensive and large-scale studies are still needed to confirm our findings.