Introduction
Since the decline in hepatitis C virus (HCV) prevalence, especially after the era of direct acting antiviral agents, metabolic associated fatty liver disease (MAFLD) has become the leading cause of chronic liver disease, with a global prevalence of 25% [1]. The term MAFLD reflects the pathophysiological features of the disease. Diagnosis depends on the presence of hepatic steatosis, plus any of the following three conditions: obesity, presence of type 2 diabetes mellitus, or evidence of metabolic deregulation [2]. MAFLD comprises two main histological phenotypes with varying prognoses: simple steatosis or steatohepatitis and fibrosis. In Western societies, prevalence of steatosis among adults is estimated to be around 3-4%, with as many as 40% of the cases progressing to advanced liver fibrosis [3]. In a young adult cohort Egyptian study, steatosis was detected in about one-third of the studied group. It confers an increased risk of diabetes, cardiovascular disease, liver cirrhosis, and cancer. At least 0.05% of adults had advanced fibrosis (F2-F3), which is the most important predictor of morbidity and mortality in MAFLD [4]. Histologic assessment of the liver biopsy is the gold standard to diagnose steatohepatitis and assess the stage of fibrosis [5, 6]. However, liver biopsy is not a routine procedure due to high costs, invasiveness, and various potential complications. There are several noninvasive scoring tools designed to assess the presence of liver fibrosis – including the Fibrosis-4 (FIB-4) score, the NAFLD Fibrosis Score (NFS), and the aspartate aminotransferase (AST) to platelet ratio index (APRI) – but data about the sensitivity and specificity of these noninvasive scoring systems are limited [7-9]. The yearly economic cost of obesity in Egypt is estimated to be 62 billion Egyptian pounds. This amount represents the expense of treating illnesses linked to adult obesity [10]. According to the 100 Million Health survey from 2019, the prevalence of obesity among Egyptian adults has risen to almost 40%, up from the 36% estimate from the STEP SMART study from 2017. Egyptian women are more likely than Egyptian men to be obese (about 50% vs. 30%, respectively). Given that women are less likely than men to engage in physical activity in Egypt, this could possibly be linked to cultural reasons [11, 12]. There is a dearth of information regarding the identification of severe and advanced liver fibrosis in Egyptian patients who are overweight or obese and undergoing bariatric surgery. Obesity places a significant financial and medical burden on Egypt [11, 12]. Hepatologists may find it easier to identify high-risk groups early on in bariatric surgery patients with MAFLD if they can identify potential risk factors for severe and extensive fibrosis. The purpose of this study was to assess the prevalence and clinical predictors of severe and advanced hepatic fibrosis (verified by liver biopsy), performed by a skilled bariatric surgeon. Furthermore, our objective was to evaluate the reliability of the previously established noninvasive fibrosis scoring methods in Egyptian MAFLD patients who were overweight or obese and had undergone bariatric surgery.
Material and methods
Patients undergoing bariatric surgery who were overweight or obese were the subject of a cross-sectional study. Patients were chosen for the trial between May and December 2022 from a university hospital’s bariatric surgery facility. Patients who satisfied the requirements for metabolic surgery and had a body mass index (BMI) of at least 32.5 kg/m2 were eligible for bariatric surgery. All participants satisfied the inclusion criteria of MAFLD established by Eslam et al. [2]. All gave written consent to a transoperative liver biopsy. The exclusion criteria for our study were: 1) overweight/obese patients who were not eligible for bariatric surgery; 2) patients who had any history of other chronic diseases (e.g. heart failure, renal failure, liver cirrhosis) or malignancy. The study was approved by our hospital ethics committee (2019-024). Written informed consent was obtained from each participant before bariatric surgery. Laboratory and clinical data were collected from the participant. Over weight and obese patients under going bariatric surgery were the subject of this cross-sectional study. Patients were chosen for the trial between May and December 2022 from a university hospital’s bariatric surgery facility. Patients who satisfied the requirements for metabolic surgery and had a body mass index (BMI) of at least 32.5 kg/m2 were eligible for bariatric surgery. The dimensionless ratio of the waist circumference to the hip circumference is known as the waist-hip ratio. determined by dividing the waist measurement by the hip measurement (women ≤ 0.8 and men ≤ 0.95) [13]. At least two of the seven criteria listed below have been identified as indicators of metabolic syndrome: 1. Obesity of the abdomen (waist circumference > 80 cm in women and ≥ 90 cm in males); 2. 130/85 mmHg or higher blood pressure, or being on an antihypertensive medication. The following conditions must be met: 3. Serum triglycerides ≥1.7 mmol/l, or using lipid-lowering medication; 4. Serum high-density lipoprotein cholesterol (HDL-c) < 1.0 mmol/l for men and < 1.3 mmol/l for women; 5. Fasting plasma glucose (FPG) ≥ 5.6 mmol/l, or medication for increased glucose; 6. HOMA IR > or equal to 2.5; 7. Plasma C-reactive protein (CRP) > 2 mg/dl [14]. Blood samples were collected preoperatively for complete blood count, prothrombin concentration (PC) and international normalization ratio (INR), liver function tests (ALT, AST, serum albumin), serum urea, serum creatinine, tests for renal functions and serum uric acid. A full lipid profile was taken, including high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, serum cholesterol and fasting blood glucose level, glycated hemoglobin HbA1c, serum uric acid, and thyroid stimulating hormone (TSH). Testing for noninvasive assessment of fibrosis was done via using generally available published parameters. The following serum liver fibrosis scores were calculated in all participants, according to previously published formulas: NFS (age, BMI, diabetic status, platelet, albumin); FIB-4 (age, ALT, AST, platelet); and APRI (AST to platelet ratio index) [7-9]. In order to measure the degree of fibrosis, bariatric surgery and intraoperative laparoscopic liver biopsies were performed on each patient (the surgeon took a wedge biopsy from the left lobe of the liver). Formalin-fixed, paraffin-embedded liver tissue specimens were routinely stained with hematoxylin-eosin. The biopsy sample needs to have ten portal tracts or more, or at least 10 mm in length. The same skilled pathologist conducted each and every histology exam. Based on the METAVIR scoring system and the steatosis, activity, and fibrosis (SAF) score, the samples were sent for histological investigation. The four phases of fibrosis were as follows: F0 denoted no fibrosis, F1 perisinusoidal or periportal fibrosis, F2 perisinusoidal and portal/periportal fibrosis, F3 bridging fibrosis, and F4 cirrhosis. Analysis using histopathology was done [15, 16]. Liver fibrosis is considered significant if it is greater than or equal to F2 and stage F3-4 is considered to be advanced fibrosis.
Statistical analysis
The collected data were coded, tabulated, and statistically analyzed using IBM SPSS Statistics software version 24. Descriptive statistics were calculated for parametric quantitative data using the mean, standard deviation and minimum and maximum values, for non-parametric quantitative data using the median, and for categorical data using the number and percentage. Analyses were performed for parametric quantitative data between the groups using the independent t-test, and for non-parametric quantitative data using the Mann-Whitney test. Statistically significant independent variables (p < 0.05) were introduced into a multivariable logistic regression (backward selection method). Analyses were conducted for qualitative data using the chi square test (if less than 20% of cells had an expected count less than 5) or Fisher’s exact test (if more than 20% of cells had an expected count less than 5). Correlation between different variables was done using Spearman’s rho correlation coefficient. ROC (receiver operating characteristic) curve analysis was carried out to determine AUC, optimal cutoff point, sensitivity, positive predictive value (PPV), negative predictive value (NPV), and accuracy. The odds ratio (OR) with a 95% confidence interval (CI) was calculated. The level of statistical significance was defined as p < 0.05.
Results
Ninety-eight individuals had bariatric surgery in 2022 from May to December; 13 were excluded from our study. As can be seen in Table 1, a total of 85 patients were included in the trial, of whom 75 (88%) were female and 11 (12%) male. Those with insignificant fibrosis (F0-F1) numbered 50 (58.8%); 44 female and 6 male. Their mean ±SD age was 38.6 ±9.1. Those with significant fibrosis (F2) numbered 24 (28.2%), 20 female and 4 male. Their age was 44.3 ±8.9. Those with advanced fibrosis (F3-F4) numbered 11 (12.9%); all of them were female. Their age was 47.4 ±4.2. Smoking and elevated BMI are significantly more prevalent in those with advanced fibrosis (p < 0.001). Waist and hip circumferences were higher in those with F2 fibrosis but not significant (p > 0.05) while waist-hip ratio was significantly higher in those with advanced fibrosis (p < 0.001). Type 2 diabetes mellitus (T2DM) and hypertension (HTN) were significantly more prevalent in those with advanced fibrosis, 100%, 81.8%, than those with significant (F2) fibrosis, 83.3%, 16.7% and those with insignificant (F0-F1) fibrosis, 28%, 16% respectively. Also fasting blood glucose and HbA1c were significantly higher in patients with advanced fibrosis than those with significant (F2) fibrosis and those with insignificant (F0-F1) fibrosis. There were no significant differences regarding the HB level, prothrombin time (PT), prothrombin concentration (PC), INR, serum albumin, TSH, urea, creatinine, LDL or HDL between such three types of patients. There were significant differences regarding total leucocytic count, platelet count, ALT, AST, serum uric acid, serum cholesterol and serum triglycerides between patients with advanced liver fibrosis, those with significant liver fibrosis and those with insignificant liver fibrosis, as shown in Table 1. FIB-4 was higher in obese patients with advanced liver fibrosis, 1.7 ±0.3, than those with significant liver fibrosis, 1.5 ±0.6, and those with insignificant liver fibrosis, 0.9 ±0.4. NFS was significantly higher in patients with advanced liver fibrosis, 2.3 ±1.8, than those with significant liver fibrosis, 0.6 ±2.4, and those with insignificant liver fibrosis, 0.2 ±1.6. APRI score was significantly higher in patients with advanced liver fibrosis, 0.6 ±0.2, than those with significant liver fibrosis, 0.4 ±0.2, and those with insignificant liver fibrosis, 0.2 ±0.1. Table 2 shows that in univariate logistic regression analysis significant and advanced liver fibrosis are significantly related to age, body mass index, smoking, waist-hip ratio, hypertension, diabetes mellitus, low platelet count, serum uric acid, FIB-4, NFS and APRI score (p < 0.05). In multivariate analysis, age (OR = 1.36, 95% CI: 1.107-1.67, p = 0.003) and serum uric acid (OR = 18.7, 95% CI: 3.37-103.8, p = 0.001) were found to be independent predictors of significant and advanced liver fibrosis.
Table 1
Demographic and laboratory data of patients with no fibrosis (F0-F1), significant fibrosis (F2), and advanced fibrosis (F3-F4)
Table 2
Logistic regression of fibrosis (≥ F2)
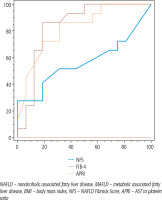
Figure 1 and Table 3 show that at a cut-off value > 3.085 NFS could detect significant liver fibrosis; its sensitivity is 27.5%, its NPV is 60.4% with 100% specificity, its PPV is 100% and AUROC 0.569, p = 0.371. At a cut-off value > 1.18 FIB-4 could detect liver fibrosis; its sensitivity is 82.8%, its negative predictive value is 87% with 84% specificity, its positive predictive value is 78.4% and AUROC is 0.879, p < 0.001. At a cut-off value > 0.316 APRI score could detect liver fibrosis with sensitivity 71.4%; its negative predictive value is 80.8%, with 84% specificity; its positive predictive value is 75.8% and AUROC is 0.790, p < 0.001.
Table 3
NFS, FIB-4, and APRI scores for the detection of significant fibrosis (F > 2)
Discussion
Hepatic fibrosis occurrence in MAFLD patients has recently attracted a lot of attention, particularly in patients undergoing bariatric surgery, however there is currently little research about the prevalence and determinants of hepatic fibrosis in those patients. In this study, we have found that about one-third or more MAFLD patients undergoing bariatric surgery showed a degree of fibrosis in liver biopsy. Among these patients, the corresponding frequencies of substantial and advanced fibrosis were 28% and 12%. There is a disparity in the incidence of fibrosis revealed by liver biopsy between studies, which may be attributed to variations in pathologists’ findings, disparities in histological scoring systems, selection bias, and epidemiological variations [17-19].
A recent study estimated that the prevalence of fibrosis is 31.6% in young Egyptian adults, which was identical to the prevalence of fibrosis in Middle Eastern populations. Udelsman et al. reported 29.3% prevalence of fibrosis among MAFLD patients in his study on non-Hispanic patients [18]. Pais et al. found that significant fibrosis is the main risk factor for cirrhosis and HCC in MAFLD patients and advanced fibrosis can persist or improve following bariatric surgery [20]. Our study reported that age, smoking, elevated WHR, T2DM (FBS, HbA1c), and hypertension are risk factors for significant fibrosis. This is in line with several studies [21-26]. Among the laboratory investigation for the studied group, our study found that elevated leucocytic count, ALT, AST, serum uric acid, triglyceride, cholesterol, and low platelet level were associated with significant fibrosis. This is in agreement with results of these studies [27-30]. Our study found that advanced age, smoking, higher BMI, elevated waist-hip ratio, T2DM, hypertension, low platelet level, elevated serum uric acid, FIB-4, APRI TEST and NFS were the main predictors of significant fibrosis. This is in line with several studies. It is in agreement with Zambrano-Huailla et al. [32].
In this study, we found that FIB-4 and APRI scoring systems are sensitive and specific for assessment of liver in fibrosis in MAFLD before bariatric surgery while NFS is not sensitive but highly specific for detection of liver fibrosis. Additionally, we discovered that although the NFS score showed poor predictive performance in comparison to the aforementioned measures, FIB-4 and APRI demonstrated sufficient AUROC (> 0.70) for predicting severe fibrosis. Huang et al. discovered that, in comparison to the NFS and BARD score, the non-invasive models, APRI, FIB-4, and Hepamet fibrosis scores (HFS), offered improved accuracy for predicting severe fibrosis [31]. According to Angulo et al., a straightforward scoring system called NFS effectively distinguishes between individuals with NAFLD who have progressed fibrosis and those who have not, hence in a significant number of cases, a liver biopsy is not required to diagnose advanced fibrosis [7]. In conclusion, our study showed that more than one-third of MAFLD patient undergoing bariatric surgery has significant fibrosis. Increasing age, smoking, high BMI, WHR, T2DM, hypertension, low platelet count, higher serum uric acid, and non-invasive scores (FIB-4, NFS, and APRI) are risk factors for significant fibrosis. The FIB-4 test and APRI score can be used as non-invasive tests to assess liver fibrosis in those with MAFLD who are candidates for bariatric surgery.