A 58-year-old female patient was admitted to our hospital with obstruction of the large intestine and she underwent an exploratory laparotomy. The findings of the laparotomy were peritoneal carcinomatosis and a large solid mass in the pelvis, probably arising from the sigmoid, causing intestinal obstruction. A colectomy was performed with removal of the mass. In the postoperative course, an enterocutaneous fistula with low output occurred, due to an injury to the third portion of the duodenum (Figure 1). The histological examination identified a neuroendocrine tumor of the large intestine, grade II, and the patient received treatment with somatostatin (Figures 2 A, B). The enterocutaneous fistula was treated conservatively and a covered self-expandable duodenal stent was placed in the third portion with progressive reduction of the output (Figure 3). The patient was safely discharged, but 62 days after she was admitted to the emergency department with hematemesis, hematochezia, and hemodynamic instability. The vital signs were blood pressure (BP) 70/45 mm Hg, bpm 120/min, SaO2 94%, a temperature of 38°C, and the patient was pale and disoriented. The laboratory studies revealed elevated white blood cells 17,800 and hemoglobin 5 g/dl. An esophagogastroduodenoscopy (EGD) was immediately performed, which revealed stent migration distally and active bleeding from the third portion. The diagnosis of primary aortoenteric fistula (PAEF) was confirmed with computed tomography (CT) angiography, where intravenous contrast extravasation to the lumen of the duodenum was recognized (Figures 4 A, B). The patient did not have a previous history of an abdominal aortic aneurysm. The endovascular approach was decided due to the hemodynamic instability of the patient and an aortic stent was placed by the interventional radiologists in the celiac aorta, behind the third portion of the duodenum with immediate cessation of bleeding and stabilization of the patient (Figures 5 A, B). Regarding the short-term outcomes, the patient did not present recurrence of the bleeding, the migrated duodenal stent was removed 20 days after the management of the PAEF and she was safely discharged with an enterocutaneous fistula without receiving home antibiotics. A close follow-up was maintained and ten months after the endovascular repair there were no signs of re-bleeding. During this period, the patient was admitted twice with fever and bacteremia, although without confirming the aortic stent as a possible source of the infection.
Figure 1
Upper gastrointestinal series showing the enterocutaneous fistula with oral-contrast leakage in the third portion of the duodenum (blue arrow)
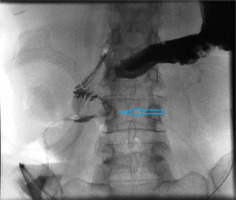
Figure 2
A – The tumor is composed of solid nests, ribbons, and gland-like structures in a dense fibrous stroma. B – The tumor cells are uniform with an organoid pattern. No mitoses are seen
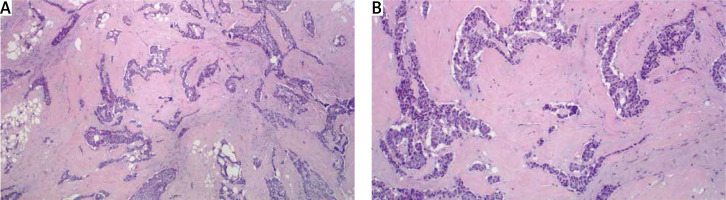
Figure 3
Treatment of the enterocutaneous fistula in the duodenum with a self-expandable covered stent (blue arrow)
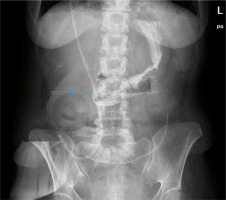
Figure 4
A – CT angiography showing the intravenous contrast extravasation in the lumen of the duodenum (red arrow). B – The migrated duodenal stent is also recognized (red arrow)
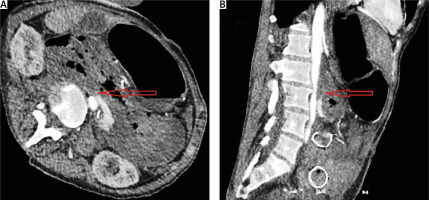
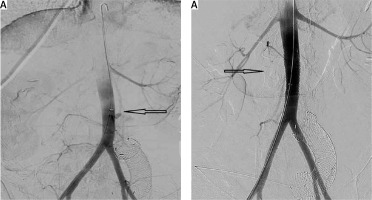
Figure 5
A, B – Extravasation of the IV contrast during the aortography and occlusion after the aortic stent placement (black arrows). The migrated duodenal stent is also recognized
The use of stents for treating duodenal injuries, especially in patients with a hostile abdomen, is an alternative and minimally invasive option, although the long-term outcomes are still not clarified. The presence of PAEF after an eroding duodenal stent is a very rare complication and the exact mechanisms remain poorly understood [1]. PAEF is associated with high morbidity and mortality and the cornerstone for survival is high clinical suspicion and immediate treatment. According to the literature, 70% of PAEF present with upper gastrointestinal hemorrhage manifested as hematochezia, hematemesis, or melena. On the other hand, the classic triad of symptoms, abdominal pain, hemorrhage, and palpable abdominal mass is found only in 23%. Another essential characteristic of PAEF is herald bleeding when an intermittent hemorrhage with spontaneous closure of the aortoenteric fistula by a thrombus is followed by a massive hemorrhage with the collapse of the patient. This diagnostic sign should always raise suspicions for the presence of an aortoenteric fistula [2]. For the diagnosis of PAEF, EGD is necessary in order to exclude other causes of GI bleeding, although it has a 25% detection rate for PAEF [3]. In contrast, CT angiography is the ideal examination, not only because it has a high detection rate of 61%, but also because it is a non-invasive method without danger of dislodging a thrombus and rebleeding [4]. Signs in the CT indicating PAEF are bowel thickening, free air in the aorta or the aneurysmatic sac, and intravenous contrast extravasation in the intestinal lumen [3]. The first successful endovascular repair of an aortoenteric fistula was described in 1997 and then the rapid development of interventional radiology established the endovascular approach as an optimal tool, supplanting the traditional open approach which was considered as the gold standard [5]. Endovascular repair is related to lower perioperative morbidity and mortality, and shorter hospital length of stay, and it leads to immediate stabilization of the patient with control of the bleeding. Critically ill patients, patients with a hostile abdomen due to previous surgery, or patients with numerous comorbidities seem to benefit from an endovascular approach and for these patients it should be considered a premium option. Despite these short-term advantages, endovascular repair is associated with re-bleeding and recurrent infections at a rate as high as 44% and for this reason it is considered a “bridge to surgery” and not a definite treatment [6]. Regarding the long-term outcomes for endovascular repair of PAEF, the data are scarce and more studies are required to identify whether an endovascular repair can stand as a sole procedure for certain patients, who are poor candidates for open surgery.






