Introduction
Gastric cancer is the fourth most commonly diagnosed malignancy and the second leading cause of cancer death worldwide. Although survival is increased by combinations of perioperative chemotherapy, and adjuvant therapies, and surgical resection, the prognosis in gastric cancer remains poor [1]. The tumor, nodule, and metastasis (TNM) classification system is highly important in treatment planning and prognosis prediction in patients with gastric cancer. However, important prognostic factors such as age, sex, location of the primary tumor, the Lauren classification, and presence of lympho-vascular invasion are not covered by the TNM classification. Moreover, biomarkers that might aid in prognosis prediction are not covered in this classification [2, 3]. Lactate dehydrogenase (LDH) and albumin are key markers of systemic inflammation and have been shown to have prognostic value in the prediction of cancer progression and metastasis in numerous cancers such as gastric cancer [4–7]. Moreover, LDH levels correlate with tumor burden and can reflect the tumor’s growth and invasive potential. It has been reported that abnormal serum albumin is closely related to the progression of many diseases. Additionally, low serum albumin levels have been shown to be associated with poor prognosis in various cancers including esophageal, gastric, pancreatic, and colorectal cancers [3, 5, 8–12].
The LDH-to-albumin ratio (LAR) is a prognostic tool used in cancer patients. Elevated LAR levels have been associated with poor prognosis in esophageal and hepatocellular cancers [13, 14]. Nevertheless, to our knowledge, there are a limited number of studies reporting on the prognostic value of LAR, and there has been no study specifically reporting on the effect of LAR on prognosis in patients with gastric cancer. The aim of this study was to assess the prognostic value of LAR in patients with gastric cancer after curative resection.
Material and methods
Study population and ethics statement
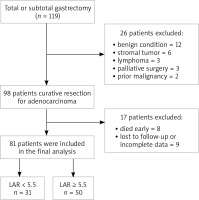
The retrospective study included 81 patients who underwent total or subtotal gastrectomy + D2 lymphadenectomy at Dicle University School of Medicine General Surgery clinic between June 1, 2013 and June 30, 2019. Figure 1 presents the flowchart of patient selection and study design. The study was approved by Dicle University Human Ethics Committee. Inclusion criteria were as follows: 1) a pretreatment diagnosis of adenocarcinoma established by endoscopy, 2) laboratory parameters including LDH and albumin levels, measured during diagnosis, 3) an R0 resection, and 4) complete clinicopathologic characteristics and follow-up data. Exclusion criteria were as follows: 1) history of treatment for cancer, 2) any form of acute or chronic inflammatory diseases or infections, 3) any form of systemic diseases, 4) presence of hemolysis, and 5) surgical resection other than R0 resection.
Data collection
Main clinical characteristics such as age, gender, Eastern Clinical Oncology Group performance status (ECOG PS), serum levels of LDH, albumin, carcinoembryonic antigen (CEA), carbohydrate antigen 19–9 (CA 19–9) differentiation, and TNM stage were retrieved from retrospective medical records. Routine laboratory measurements including white blood cell (WBC), neutrophil, lymphocyte, and platelet counts were performed before treatment (since surgery and chemotherapy will change the LDH value). The LDH value was correlated with the aspartate aminotransferase (AST), alanine aminotransferase (ALT), and potassium values and was excluded from the study in the presence of hemolysis. Presence of leakage was confirmed by a combination of clinical, radiological, endoscopic features and surgical exploration findings [15]. Metastatic lymph node ratio (rN) was defined as the number of metastatic lymph nodes divided by the total number of lymph nodes evaluated and was divided into four categories (0%, 1–20%, 21–50%, and ≥ 51%) [16]. Tumor staging was performed according to the Union for International Cancer Control-American Joint Committee on Cancer (UICC-AJCC) TNM Classification System 7th Edition [17].
Follow-up
All the patients were followed up every 3 months until 2 years after surgery, then every 6 months for up to 5 years, and then every year or until death [18]. Presence of recurrence was confirmed by clinical, radiological, endoscopic, and biopsy findings (when needed) and by surgical exploration. Disease-free survival (DFS) was defined as the time interval between the date of operation and the time when recurrence was first identified. Overall survival (OS) was defined as the time interval from the date of surgery to the date of death. For patients without any sign of an event, the last follow-up data constituted the terminal record.
Cutoff determination of LAR
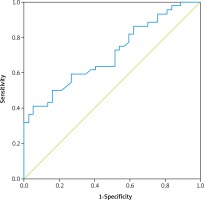
A time-dependent receiver operating characteristics (ROC) curve analysis was performed to predict patients who died before the median OS and the analysis revealed that the optimal threshold for pretreatment LAR was 5.5 (sensitivity, 68.2%; specificity, 48.6%; area under the ROC curve [AUC]: 0.706, p = 0.001) (Fig. 2). On the other hand, the cutoff values determined for the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were 2.25 and 134.09, respectively.
Fig. 2
Determination of the best cut-off value for pretreatment lactate dehydrogenase-to-albumin ratio (LAR). Time-dependent receiver operating characteristic (ROC) curve for pretreatment LAR. The optimal threshold for pretreatment LAR was determined as 5.5 (sensitivity, 68.2%; specificity, 48.6%; area under the ROC curve [AUC]: 0.706, p = 0.001)
Statistical analysis
Data collected within the scope of the study were analyzed using IBM SPSS Statistics version 21 for Windows software (IBM Corp, Armonk, NY, USA). Continuous variables were expressed as means with standard deviations (SD) or medians with ranges. Categorical variables were expressed as frequencies with percentages. For group comparisons (LAR < 5.5 vs. LAR ≥ 5.5), the χ2 test or Fisher’s exact test (categorical variables) and the independent sample t-test (continuous variables) were used to compare the differences between subgroups. The overall survival was estimated using the Kaplan-Meier method and the log-rank test was used for the comparison of the outcomes. A Cox regression model was used to analyze the independent prognostic risk factors. Significant factors identified in univariate analysis were subsequently included in the multivariate Cox proportional hazard model. A two-tailed p < 0.05 was considered statistically significant.
Results
The 81 patients comprised 55 (67.9%) men and 26 (32.1%) women with a mean age of 60.2 ±13.8 (range, 29–87) years. LAR was < 5.5 in 31 (38.2%) and ≥ 5.5 in 50 (61.7%) patients. Table 1 presents the demographic, clinical, and pathological characteristics of the patients in both groups. Mean LDH level was 210.9 ±38.6 U/l and 163.2 ±22.6 U/l and mean albumin level was 30.8 ±4.76 g/l and 36.7 ±5.29 g/l in patients with LAR ≥ 5.5 and LAR < 5.5, respectively, and these differences were statistically significant (p < 0.001 for both). However, the demographic, clinical, and pathological characteristics of the two groups were similar. Mean follow-up period was 27 (range, 6–78) months and the follow-up periods were also similar in both groups. Throughout the study period, 44 (54.3%) patients died of gastric cancer and the median OS time was 34.8 and 45 months in patients with LAR ≥ 5.5 and LAR < 5.5, respectively (p = 0.278, Fig. 3).
Fig. 3
Kaplan-Meier survival curves for gastric cancer patients. Comparison of survival outcomes between patients with pretreatment lactic dehydrogenase-to-albumin ratio (LAR) ≥ 5.5 (n = 50) and pretreatment LAR < 5.5 (n = 31). The 5-year overall survival rate was 34.8% and 45.0% in patients with LAR ≥ 5.5 and LAR < 5.5, respectively (p = 0.278)
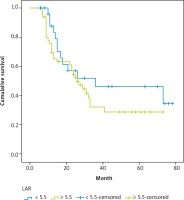
Table 1
Relationship between lactic dehydrogenase-to-albumin ratio (LAR) and clinical and pathological parameters
[i] PS – performance status, ECOG – Eastern Clinical Oncology Group, LDH – lactate dehydrogenase, PLR – platelet-to-lymphocyte ratio, NLR – neutrophil-to-lymphocyte ratio, CEA – carcinoembryonic antigen, CA 19–9 – carbohydrate antigen 19–9, LVI – lymphovascular invasion, DFS – disease-free survival, * differences between the groups with χ2 test are statistically significant p < 0.05
Univariate analysis was performed to determine the prognostic value of LAR and other clinical variables for OS (Table 2) and the analysis indicated that NLR ≥ 2.25 (p = 0.048), tumor stage (p = 0.019), N2–3 (p = 0.001), lymphovascular invasion (LVI) (p = 0.001), rN > 20 (p = 0.003), and rN > 50 (p < 0.001) were significant factors for OS, while LAR (p = 0.288) was an insignificant factor for OS. In multivariate analysis, however, only rN > 20% was revealed as an independent factor for OS and this significance level increased when the rN was higher than 50% (OR = 8.572, 95% CI: 1.555–47.252, p = 0.014) (Table 3).
Table 2
Univariate Cox regression analysis of survival outcomes in patients with gastric cancer
[i] OR – odds ratio, CI – confidence interval, ECOG PS – Eastern Clinical Oncology Group performance status, CEA – carcinoembryonic antigen, CA 19–9 – carbohydrate antigen 19–9, LAR – lactic dehydrogenase-to-albumin ratio, NLR – neutrophil-to-lymphocyte ratio, PLR – platelet-to-lymphocyte ratio, TG – total gastrectomy, LVI – lymphovascular invasion
Table 3
Multivariable Cox regression analysis of survival outcomes in patients with gastric cancer
Discussion
Serum LDH levels have been shown to be associated with tumor hypoxia, neo-angiogenesis, and poor prognosis in numerous tumor types. The oxidoreductase LDH, which converts pyruvate to lactate when oxygen is absent or in short supply, plays a crucial role in the metabolism of cancer cells. LDH-A is overexpressed in hypoxic carcinomas as well as metastatic cancer cells, and its levels correlate with tumor viability. Increased tumor burden correlates with a more aggressive cancer progression and an increased mitotic index and serum LDH level [3, 10, 19, 20]. On the other hand, serum albumin level is a significant marker of nutritional status. In patients with gastric cancer, it is recognized that there may be reduced caloric intake owing to stenosis of the cardia or pylorus. The tumor-induced systemic inflammatory response contributes to progressive loss of albumin; therefore, low albumin level is a valuable prognostic factor for poor survival in patients with gastric cancer. In addition, hypoalbuminemia has been shown to be associated with higher postoperative complication rates and longer hospital stays [11, 21, 22]. Taken together, these findings suggest that high LDH and low albumin levels will indicate a poor prognosis. In the literature, there are a limited number of studies reporting on the prognostic effect of LAR. Feng et al. [13] evaluated 346 patients with esophageal squamous cell cancer and reported that LAR, TNM stage, and weight loss were effective factors for survival. In another study, Gan et al. [14] found that increased LAR levels were associated with a poor prognosis in patients with hepatocellular cancer. In both of these studies, the cutoff value for LAR was determined as 5.5. Similarly, in our study, the cutoff value for LAR was 5.5, and although the OS decreased as the LAR increased, no significant correlation was established (p = 0.288). Further large-scale studies with larger patient series and longer follow-up periods are needed to investigate the effect of LAR on prognosis in gastric cancer.
Recurrence is the most common cause of cancer-related death in gastric cancer patients. Owing to the fact that more than half of gastric cancers are advanced at the time of diagnosis, even when a curative resection is possible, recurrence may occur in approximately 60% of patients [21]. The recurrence of surgically resectable gastric cancer is influenced significantly by the presence of lymph node metastasis. The higher the number of metastatic lymph nodes, the worse is the prognosis of gastric cancer patients [23, 24]. The resection of 15 nodes is recommended in the AJCC’s TNM staging system. Metastatic lymph node ratio (rN) is a widely accepted prognostic marker of gastric cancer, and an increased rN is considered to indicate a poor prognosis. On the other hand, the clinical significance of the rate of positive lymph nodes has recently increased due to the decreasing number of lymph nodes dissected after chemotherapy [16, 25, 26]. In the present study, multivariate analysis indicated that only rN > 20% was a significant factor for OS, and this significance level increased when the rN was higher than 50% (HR = 8.572, 95% CI: 1.555–47.252, p = 0.014). Increase in the rate of metastatic lymph node affects survival negatively.
There are several limitations associated with the present study: 1) a small number of patients were evaluated and there was selection bias, 2) the study design was retrospective, 3) no long-term follow-up data were available, and 4) the optimum cutoff value for preoperative LAR remained unknown, although 5.5 was set as the cutoff value using the results of a ROC analysis.