Introduction
Diffuse large B-cell non-Hodgkin lymphoma (DLBCL) is the largest common category of adult lymphoma [1]. B-cell lymphomas are heterogeneous tumour as regards histomorphology, clinical manifestations, immunophenotyping, and prediction of prognosis [2]. It is the commonest subtype of non-Hodgkin lymphoma, which occurs in several nodal and extra-nodal sites [3].
The standard chemotherapy protocol is Rituximab, Doxorubicin, Cyclophosphamide, Vincristine, and Prednisone (R-CHOP), which leads to about 70% complete remission [4]. Recurrence and treatment resistance happened in one-third of cases, leading to the progressive disease of DLBCL after treatment [5]. Myeloid differentiation factor 88 (MYD88) was recently found to play a role as a disease-related main gene, an adaptor-soluble cytoplasmic protein that is intended in signal inflammatory pathways downriver followers of interleukin (IL-1) and toll-like receptor [6], and playing a main role in native immunity [7]. Myeloid differentiation factor 88 was recently found to play roles in the molecular classification of DLBCL cases, particularly cases that have worse prognosis [8], which points to the possibility of using it as prognostic parameter that could help in the detection of targeted therapy [9].
Transducin (β)-like (1) X linked receptor 1 (TBL1XR1) is the central element of the NCoR/SMRT dictation co-repressor complex. The prognostic values of TBL1XR1 as a tumour progression biomarker was recently pointed out in many cancers [10], including cervical cancer [11], breast cancer [12], nasopharyngeal carcinoma [13], hepatocellular carcinoma [14], digestive cancers [15–17], and ovarian cancer [18]. The prognostic roles of both MYD88 and trans-ducin β-like receptor-1 (TBLR1) expression have not been sufficiently assessed in DLBCL patients.
In the present study we aimed to assess the clinicopathological correlation and prognostic roles of both MYD88 and TBLR1 expression in tissues of DLBCL patients using immunohistochemistry (IHC) (Table 1, 2).
Table 1
Clinical outcome of patients in correlation with transducin (β)-like receptor 1 and myeloid differentiation factor 88 expression
Table 2
Overall and relapse free survival analysis of patients in correlation with transducin (β)-like receptor 1 and myeloid differentiation factor 88 expression
Material and methods
The current prospective study included tissues derived from 100 patients with DLBCL, who were admitted and treated in Medical Oncology, Clinical Oncology, Nuclear Medicine, and Internal Medicine Departments, Faculty of Medicine, Zagazig University hospitals in the period from August 2016 and July 2019. The cases were diagnosed by excisional biopsy in the General Surgery Department, and samples were sent to Pathology Department where they were processed, diagnosed, and graded.
Treatment protocols were R-CHOP, CHOP, R-CVP, CVP, or best supportive care only ± involved field radiotherapy (Table 3, 4).
Table 3
Treatment plan and response to therapy of the studied group
Table 4
Treatment plan in correlation with transducin (β)-like receptor 1 and myeloid differentiation factor 88 expression
Inclusion criteria
All cases diagnosed with DLBCL (NOS) CD20 +ve after histopathological and immunohistochemical confirmation according to the World Health Organization (2016) diagnostic principles of lymphoid and hematopoietic tumour were included [19].
Approval from the local Ethics Committee and written informed consent from all included patients were acquired. Presentation and follow-up data were collected from patients’ files.
Exclusion criteria
Patients diagnosed with other histopathological subtypes of non-Hodgkin lymphoma, cases diagnosed with primary mediastinal large B-cell lymphoma, HIV-related lymphoma, primary central nervous system lymphoma, special morphologic DLBCL subtypes (e.g. anaplastic subtype), positive EBV lymphoma, patients with incomplete data, and patients lost to follow-up were excluded.
Immunohistochemistry
The tissue samples from 100 cases with DLBCL were incubated with primary monoclonal anti-MYD88 and TBLR1 antibodies (cat. no. ab 133739 and ab 117761, respectively; Abcam, U.S.A, dilution 1 : 200).
Myeloid differentiation factor 88 was confined to the cytoplasm of lymphoma cells. Staining intensity scores were recorded as negative: 0, weak: 1, moderate: 2, and intense: 3. The staining extent scores were recorded as 0: 0% of tumour cell stained 1: < 10%, 2: 10 –50%, and 3: > 50%. Then we summed the 2 scores to give a total score from 0 to 6. The score (0, 1) represents negative and (2–6) represents positive MYD88 expression [9].
Transducin (β)-like receptor 1 was confined to the nuclei of lymphoma cells, the staining intensity scores were recorded as (0: negative, 1: weak, 2: moderate, and 3: strong), and the extent of tumour cell staining scores were recorded as 0 ≤ 20%, 1: 21–50%, 2: 51–80%, 3: 81–100%. The total score was found by multiplying the percentage score with the intensity score, giving a result of 0–9. A score of IHC less than 4 was assumed as low TBLR1 expression. Scores more than or equal to 4 were assumed as high TBLR1 expression [10].
Due to different localizations of both markers in malignant lymphocytes, we used different evaluation methods and different cut-off points (Fig. 1).
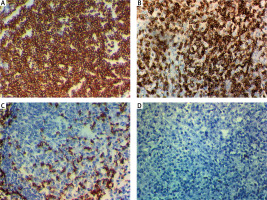
Fig. 1
Expression of myeloid differentiation factor 88 (MYD88) in the cytoplasm of cells of primary diffuse large B-cell non-Hodgkin lymphoma (DLBCL). High cytoplasmic expression of MYD88 in DLBCL; stage IV × 400 (A), high cytoplasmic expression of MYD88 in DLBCL; stage III × 400 (B), low cytoplasmic expression of MYD88 in DLBCL; stage II × 400 (C), negative cytoplasmic expression of MYD88 in DLBCL; stage I × 400 (D)
Statistical analysis
The collected information was computerized then analysed by using Statistical Set for Social Sciences (24 Inc., SPSS, Chicago, IL, U.S.A.). Information was verified by normal dispersal using the Shapiro-Wilk test. Fisher exact and chi-square (χ2) tests were performed to estimate difference among the variable quantities, as shown in Figure 2.
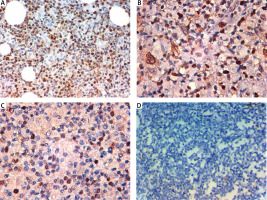
Fig. 2
Expression of transducin (β)-like receptor 1 (TBLR1) in the nuclei of cells of primary diffuse large B-cell non-Hodgkin lymphoma (DLBCL). High nuclear expression of TBLR1in DLBCL; stage IV × 400 (A), high nuclear expression of TBLR1 in DLBCL; stage III × 400 (B), low nuclear expression of TBLR1 in DLBCL; stage II × 400 (C), negative nuclear expression of TBLR1 in DLBCL; stage I × 400 (D)
Survival analysis
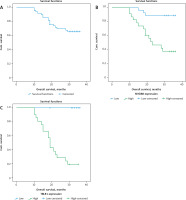
The Kaplan -Meier method was performed for evaluation of overall and event-free survival. The log rank test was used for the survival curves. Overall survival (OS) was designed as interval among information of date of latter follow-up, diagnosis until date death or study end. Relapse-free survival (RFS) was estimated from the time of documented remission to the date of documented disease relapse or study end. Model of Cox hazards proportional was performed for univariate analysis. Variable quantities with statistically significant of univariate analysis were involved in multivariate model of Cox proportional hazards. A p-value ≤ 0.05 specified significance, while p < 0.001 specified a highly significant difference (Fig. 3–5).
Fig. 3
Kaplan-Meier survival curves of 3-year relapse-free survival (RFS) rate and 3-year overall survival (OS) rate of patients with primary diffuse large B-cell non-Hodgkin lymphoma (DLBCL). RFS rate stratified according to treatment regimen (A), OS rate stratified according to treatment regimen (B)
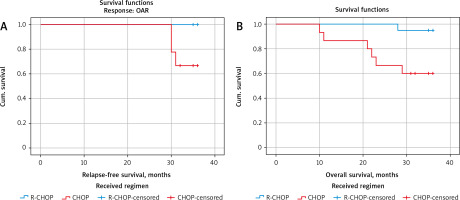
Fig. 4
Kaplan-Meier survival curves of 3-year relapse-free survival (RFS) rate of patients with primary diffuse large B-cell non-Hodgkin lymphoma (DLBCL). RFS rate of all included DLBCL patients (A), RFS rate stratified according to myeloid differentiation factor 88 expression in tissues of included DLBCL patients (B), RFS rate stratified by transducin (β)-like receptor 1 expression in tissues of included DLBCL patients (C)
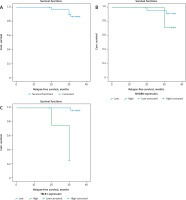
Fig. 5
Kaplan-Meier survival curves of 3-year overall survival (OS) rate of patients with primary diffuse large B-cell non-Hodgkin lymphoma (DLBCL). OS rate of all included DLBCL patients (A), OS rate stratified according to myeloid differentiation factor 88 expression in tissues of included DLBCL patients (B), OS rate stratified by transducing (β)-like receptor 1 expression in tissues of included DLBCL patients (C)
Results
Clinicopathological parameters and associations with included marker expressions are shown in Table 5.
Table 5
Clinicopathological parameters of patients in correlation with transducin (β)-like receptor 1 and myeloid differentiation factor 88 expression
The 100 DLBCL patients included 62 males and 38 female patients with age ranges 40 to 70 years. Stage I was represented in 22 cases, stage II was found in 32 cases, stage III was found in 26 cases, and stage IV was found in 20 cases (Table 5).
Immunohistochemical results
Myeloid differentiation factor 88 expression
High cytoplasmic MYD88 expression in tumour cells was found in 46% (46/100), and it was significantly associated with older age, bone marrow involvement, presence of extra-nodal extension, presence of bulky nodes, and advanced stage of DLBCL cases (p < 0.001).
No significant association was found between MYD88 expression and sex.
Transducin (β)-like receptor 1 expression
High nuclear TBLR1 expression in tumour cells was found in 42% (42/100) of cases, and it was significantly associated with older age, bone marrow involvement, presence of extra-nodal extension, presence of bulky nodes, and advanced stage of DLBCL cases (p < 0.001).
No significant association was found between TBLR1 expression and sex.
There is a positive association between both MYD88 and TBLR1 expression in tissues of DLBCL patients (p < 0.001).
According to univariate analysis, TBLR1 and MYD88 expression and age were independent prognostic factors, while only MYD88 expression was an unrestrained prognostic factor according to a multivariate analysis hazard ratio of 4.8 (1.6–14.0), with a confidence interval that positively correlated with OS (Table 6).
Table 6
Univariate and multivariable analyses for overall and relapse-free survival
Patients with higher TBLR1 and MYD88 expression have higher incidence of disease recurrence and progression, and unfavourable RFS and OS rates (p < 0.001), as the 3-year RFS was 91.5% in patients with low MYD88 expression while it was 71.4% in high expression patients (p = 0.005). Patients with high MYD88 expression had shorter 3-year OS compared to those with low expression (37.5% vs. 88.9%, respectively) (p < 0.001).
Also, patients with high TBLR1 expression had poorer 3-year RFS compared to low-expression patients (25% vs. 96.3%, respectively – p < 0.001) and shorter 3-year OS (19% vs. 100%, respectively – p < 0.001).
Discussion
The prognostic mutations and many precipitated genes in DLBCL have been recognized in recent years. The expression of their encoded proteins with a probable relationship to patient outcome is mainly unknown.
In the study by Niu et al. [1] the MYD88 expressions in DLBCL were examined by performing immunohistochemical methods to evaluate MYD88 protein expression. Limited research has been performed by immunohistochemical method to detect expression of MYD88 protein.
It was previously found that overexpression of MYD88 protein was in 38.7% of DLBCL patients [20]. Those results were consistent with our study finding (38%). Also, MYD88-positive expression was associated with survival status.
The higher Bcl-2 expression was associated with positive MYD88 protein expression in the Niu et al. [1] study, which might show that Bcl-2 and MYD 88 expression impede apoptosis of tumour cells, encourage its proliferation, augmenting the other oncogenes’ role in lymphoma cells. The progress of lymphoma is accelerated by Bcl-2 protein, and it encourages lymphoma cell resistance to chemotherapy drugs [21]. Positive expression of MYD88 was positively associated with higher Ki-67 expression. This conclusion suggests that MYD88 protein expression might impede apoptosis of cancer cells and encourage its proliferation [1].
According to our study, we observed that MYD88 expression in DLBCL patients correlated to their survival status, and high expression correlated to low OS and RFS. These findings are in agreement with [1, 22] that provided MYD88 (L265P) mutation is related to the worse prognosis of DLBCL cases who already treated with typical R-CHOP immunochemotherapy. Nevertheless, other studies have established that mutation of MYD88 protein expression is not associated with OS rates of lymphoma cases [23].
In prior studies, MYD88 (L265P) mutation was observed in DLBCL cases (6.5–19%) [6, 22]. In the Niu et al. [1] study, MYD88 (L265P) mutation was observed in DLBCL cases (29%). Also, this genetic mutation was positively associated with Eastern Cooperative Oncology Group scores; the high score (72.4%) had high mutation rates compared with the low score (27.6%). The Eastern Coope-rative Oncology Group score was a guide performed for appreciate tolerance to treatment, general health status, and patients’ physical status. It was previously found that MYD88 (L265P) gene mutation of lymphoma patients that observed MYD88 (L265P) mutation was associated with immune-phenotyping, prognostic outcome, and age, but this mutation was not correlated with sex and stage [24]. The preceding findings showed that genetic mutation MYD88 (L265P) of was related to the staging (Ann-Arbor), which was related to worse prognosis [1].
The main role of MYD 88 protein in NF-κB pathway is that its higher expression can cause the aberrant stimulation of this pathway even though activation of NF-κB pathway continues the proliferation DLBCL cells [25]. The gene mutation of MYD88 has great significance in the assessment of the progression and prognosis of DLBCL cases, signifying that this is of great value as an immunotherapy target [26].
Transducin (β)-like receptor 1 is a silencing mediator in retinoic acid with thyroid hormone receptor (SMRT/NCo R), which plays a role in a transcriptional repression and triggering NF-κB signalling activation. Diffuse large B cell lymphoma depends on activation of NF-κB pathway [25]. This mechanism can describe the augmented violence of lymphoma, detected in cases with higher TBLR1 protein expression. In [27] it was found that protein expression of TBLR1 has value in the assessment of prognostic outcomes and progression in DLBCL cases. According to our study, high TBLR1 expression was associated with poor RFS and OS. Our results are consistent with those in the study by Ednersson et al. and Schmitz et al. [27, 28]. Also, expression of TBLR1 protein evaluated by immunohistochemical method is correlated with worse outcome of cervical cancer [29], serous ovarian carcinoma [10], and gastric cancer [15]. The TB1R1 protein expression also encourages invasion and migration of ovarian tumour cells [18].
Conclusions
The immunohistochemical expressions of MYD88 protein and TBLR1 protein were correlated with shortened OS and RFS rates and progression in DLBCL cases.
The limitations of our study are the small samples sizes, and the fact that we should use molecular ways for better assessment of gene mutation in DLBCL cases.








