Summary
In this research, I have focused on how to prolong right ventricular outflow tract (RVOT) stenting efficiency and designed a semi-prospective study. I may say this is a unique study that demonstrates long-term treatment with propranolol given after RVOT stenting in tetralogy of Fallot (ToF) and variants. I found that, particularly under 3 months of age, it may reduce the need for further interventions prior to complete repair. As most centers use RVOT stenting in the management of ToF and variants as first-stage palliation, this should be highlighted in further studies after this publication.
Introduction
In developing countries, initial palliations during early infancy are frequently practiced in patients with tetralogy of Fallot (ToF) and variants. Total correction surgery was most commonly performed at around 1 year of age due to various limitations [1–3].
As a consequence of this policy, one-quarter of patients with ToF and variants need initial palliation. Palliation was frequent at a median age of 3 months and 40% underwent palliation during the neonatal period [1, 4].
In this context, right ventricular outflow tract (RVOT) stenting is a valuable treatment option for selected patients with severe RVOT obstruction. However, this palliative treatment option requires reintervention until total correction surgery. A few months later, the majority of patients require redilatation of the stent or a second stent due to progressive muscular infundibular stenosis [5–8].
Long-term oral treatment with propranolol is generally accepted as a valuable alternative palliation for ToF and variants complicated by episodes of severe cyanosis. It reduces RV hypercontractility, increases relaxation time and peripheral vascular resistance, and allows better pulmonary blood. It has been used since the 1960s to prevent cyanotic spells [9–11].
Aim
The purpose of this study was to determine whether propranolol therapy after RVOT stenting may provide long-term benefit for these patients with ToF and variants. We hypothesized that propranolol administration after RVOT stenting would be associated with a reduction in the number of patients requiring additional interventions before total correction surgery.
Material and methods
Study design and participants
In our institution, we began using RVOT stenting in the management of ToF and variants in March 2017 as a first-stage palliation. Twenty-five cyanotic infants (oxygen saturation < 70%) under 6 months of age with ToF and variants who received palliation with an RVOT stent were included in this study.
All patients were discussed beforehand with the cardiothoracic team, and it was decided to perform RVOT stenting in patients in whom the total correction surgery could not be performed, including low-weight infants (under 7 kg) and patients with complex anatomy or hypoplastic pulmonary arteries (z-score of < −1.5).
The protocol for the research project has been approved by a suitably constituted Ethics Committee of our institution within which the work was carried out, and it conforms to the provisions of the Declaration of Helsinki in 2013. Written informed consent was obtained from all parents or other surrogates for publication of this study and any accompanying images.
In the first 11 cases (Group 1), all of them had TOF except two with a double-outlet right ventricle (DORV) and two with a complete atrioventricular septal defect (AVSD) with subpulmonary obstruction. RVOT stenting was performed, followed without propranolol medication. In the subsequent 14 cases (Group 2), all of whom had TOF except two with DORV and one with complete AVSD with subpulmonary obstruction, we administered propranolol (1 mg/kg/6 h orally) after RVOT stenting. Acetylsalicylic acid 5 mg/kg/day was commenced in both groups.
Procedures, follow-up, and monitoring
All procedures were performed under general anesthesia and mechanical ventilation.
Right femoral venous access was the preferred approach in children weighing more than 2.5 kg. In smaller children, a right internal jugular approach was frequently chosen. All patients received 50 IU of heparin/kg and standard antibiotic prophylaxis. The initial right ventricular angiogram was performed using 30° right anterior oblique with 20° cranial tilt and a straight lateral projection. Selection of the size and the type of stent to be implanted was guided by the size of the patient and the dimensions of the outflow tract. The preference was for not crossing the pulmonary valve annulus. A coronary stent (Liberte, Boston Scientific, Natick, Massachusetts) was used in all children at initial palliation and a bare metal stent (Formula, Cook Europe, Bjaeverskov, Denmark) was used in those who required reintervention after 6 months of age.
Serial measurements of branch pulmonary artery diameters at presentation, intervention, and during monthly follow-up were obtained by cross-sectional echocardiography. Angiography was not routinely performed in all cases at the time of surgery and so could not be used for uniform analysis across the series. All branch PA measurements were Z-scored against the body surface area as calculated using body weight by the Boyd equation and charted in the British National Paediatric Formulary. Z-score calculations were performed on vessel measurements prior to each intervention with the primary focus on preinterventional and presurgical right and left PA Z-scores. Z-scores were calculated using the regression equations outlined by Pettersen et al. [12].
Transcatheter reintervention was performed if the peripheral oxygen saturation fell below 75% and/or there was a branch PA Z-score of < –2 during follow-up.
Statistical analysis
Medians and IQRs were used to summarize the continuous variables. Categorical data were expressed as counts and percentages. Crude comparisons of patients undergoing either palliation method used Fisher’s exact test or the Mann-Whitney U test/Wilcoxon rank-sum test as appropriate for count and continuous variables, respectively. Survival and freedom from reintervention were estimated using the Kaplan-Meier method and the difference between survival distributions assessed using the log-rank test. The statistical software package IBM SPSS Statistics 22.0 was used for statistical data.
Results
All interventional procedures were uneventful. There was no procedural death or emergency surgery. The two groups did not differ significantly in terms of underlying cardiac anatomy or initial prepalliative management (prostaglandin infusion, β-blockers, history of hypercyanotic spells) characteristics and number of relevant noncardiac comorbidities (e.g., prematurity, abdominal abnormalities, intrauterine growth restriction) (Table I).
Table I
Patient characteristics and comparison of important patient demographics/parameters between the RVOT stenting without propranolol medication group and the RVOT stenting with propranolol medication group (p-values calculated using Fisher’s exact test and Mann-Whitney U test)
Median age at RVOT stent implantation was 92 (range: 9–175) days and median weight was 5.4 (1.7–6.4) kg. Median age and weight did not differ significantly among groups at RVOT stent implantation. Median stent diameter was 4.5 (3.7–4.9) mm and median stent length was 16 (12–24) mm. Four patients required two stents to cover the entire length of the infundibulum. All of these were in the first group of patients during the learning curve period. Coronary stent sizes (diameters and lengths) did not differ between the two groups. Median procedure time was 53 (23–260) min and median screening time was 14 (5.2–73) min. Procedure time was significantly longer in Group 1 (Table I).
There was no intraprocedural death during RVOT stenting. One patient with Down syndrome developed sepsis at 5 days after RVOT stenting and death occurred on day 43 due to multiple organ system dysfunction. This patient did not take part in this study.
The rate of catheter re-intervention after RVOT stenting was 8/25 (32%) during follow-up for recurrence of cyanosis. Reintervention was performed in 6/11 (54%) patients in Group 1 and 2/14 (14%) in Group 2. Kaplan-Meier analysis showed a significant difference in reintervention rate between groups (p = 0.041, log rank test) (Figure 1).
Figure 1
Kaplan-Meier analysis showed significant difference in reintervention rate between groups followed with or without propranolol medication after right ventricular outflow tract stenting (p = 0.041, log rank test). Furthermore, reintervention events of group followed without propranolol medication were more likely to be concentrated in the early period of initial RVOT stenting and follow-up
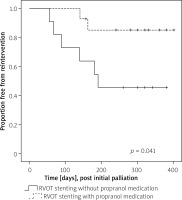
Median time to reintervention was 70 days (interquartile range: 40–140 days) in 6 patients followed without propranolol medication. The remaining 2 patients followed with propranolol medication required reintervention 150 and 170 days after initial intervention.
The median pre-palliation LPA and RPA Z-scores did not differ between the two groups. However, the median LPA and RPA Z-scores were better at the 3-month follow-up in Group 2 patients (p = 0.002 and p = 0.005, respectively). Within the entire group of patients who underwent complete surgical repair (22 patients), the median LPA and RPA Z-scores did not differ between the two groups, as shown in the Table I.
At the time of writing, total correction of ToF has been performed in 23 patients including 11 in Group 1 and 12 in Group 2. At the time of surgery, the median age was 258 days (interquartile range: 200–480 days), and the median weight was 8.2 kg.
All the patients underwent standard cardiopulmonary bypass techniques with aorto-bicaval cannulation and moderate hypothermia. Nineteen of the 22 (86%) repaired patients received a transannular patch at the time of surgery. Complete stent removal was possible in only 16 patients. In 6 patients, a small part of the stent was left in situ to avoid damage to the surrounding tissue and the margin of the VSD. The remnant of the residual stent did not appear to make placement of the VSD sutures difficult, and there were no instances of residual VSD. One patient required a right ventricle-pulmonary artery (RV-PA) conduit for anomalous LAD and, in this patient, the stent was left in situ. Three patients underwent valve-sparing correction without a transannular patch. The average bypass time was 109 ±42 min (median: 95 min) and cross-clamp time 68 ±29 min (median: 67 min). The median intensive therapy unit stay was 2 days.
Discussion
RVOT stenting in symptomatic infants with ToF and variants is an alternative to surgical creation of a modified Blalock-Taussig shunt. Stumper et al. [5] reported excellent outcomes with a concurrent series of 52 patients. The authors concluded that RVOT stenting is a viable first-line treatment option for selected patients with severe RVOT obstruction as an alternative to systemic-to-pulmonary artery shunting procedures and for whom early complete repair carries significant additional risk or complexity [5–7].
In our clinic, RVOT stenting is the palliation of choice for infants with ToF and variants who are not suitable for repair at the time of presentation. Median age at RVOT stent implantation was two and a half months in our study.
However, RVOT stenting has short-term efficacy with a single procedure, particularly prior to complete repair. In an attempt to limit early pulmonary overcirculation, the stents chosen for implantation are of rather small size, particularly during the neonatal period. Additionally, muscular infundibular stenosis progresses after RVOT stenting. Thus, reintervention is required in most patients after a median of 3 months [5, 13, 14].
Propranolol, a non-selective β-adrenergic antagonist, is of remarkable practical value in symptomatic cases of ToF and variants. The action of propranolol is to decrease the contractility of the muscular right ventricular outflow tract, and thereby may obviate the tendency for progressive hypertrophic and fibrotic changes to develop in the infundibular portion of the right ventricle. In long-term treatment, the general trend is toward the elimination of palliative procedures in children with ToF and variants [9–11].
In our series, time to complete repair after RVOT stenting was 5.5 months and the median age was 8 months at the time of surgery. Although reintervention was needed after a median of 2 months and 10 days later in the solely RVOT stent group, this was significantly prolonged (median five and a half months) in the propranolol administered group after RVOT stenting. The typical angiographic findings in those patients who developed an early RVOT reobstruction were either stenosis at the proximal stent end or progressive muscular narrowing below the stent manifesting within weeks after the initial intervention (Figure 2). Thus, reintervention was required in 6 of 11 patients with RVOT stenting followed without propranolol medication. Furthermore, 5 patients underwent initial stent implantation under 3 months of age. Median time to total correction after initial stenting was 5 months in those 5 patients. The remaining one had complete AVSD with subpulmonary obstruction and underwent initial stent implantation with 4.5 mm stents at 5 months of age. Reinterventions were needed with a 5 mm stent at 3 months after the first RVOT stenting and with a 6 mm stent at 5 months after the second RVOT stenting. Total correction was performed at 16 months of age.
Figure 2
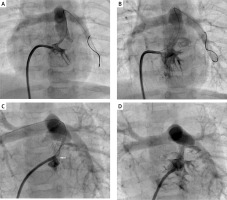
Right ventricular outflow tract (RVOT) stenting procedure in a 3.6 kg neonate with tetralogy of Fallot and recurrent stenosis occurred at the proximal stent end within 3 months of initial stenting. A – Initial right ventricular outflow tract angiogram in 30° right anterior oblique and cranial angulation. B – 4 mm 16 mm long coronary stent in position. Saturations improved from 72% to 89%. C – Progressive muscular narrowing below the stent (white arrow). D – The muscular stenosis was treated by restenting the outflow tract, with the 4.5 mm 12 mm stent covering the muscular band proximally and extending telescopically well into the prior stent. Saturations improved from 68% to 92%
However, reintervention was required in 2 of 14 patients in the propranolol administered group after RVOT stenting. Both patients had complete AVSD with subpulmonary obstruction. Total correction was performed at 14 months of age in one and 16 months in the other.
Both underwent initial stent implantation with 4.5 mm stents at 4 months of age. Reinterventions were needed with 6 mm stents in both at 7 and 8 months after the first RVOT stenting.
At the 3-month follow-up, branch pulmonary artery diameters improved more in the propranolol-administered group after RVOT stenting. Propranolol administration after RVOT stenting had a similar effect to improve branch pulmonary arteries as compared to the additional stent requiring group at the final follow-up. At the time of writing, 22 patients have undergone complete surgical repair and branch pulmonary artery diameters have improved well in both groups.
Limitations. Operative and postoperative periods were uneventful in both groups.
The small number of patients was the first limitation of our study. Secondly, the majority of the patients in Group 1 covered our learning curve, a period of time during which procedural modifications occurred, including the degree to which the infundibulum was covered. That is why 4 patients needed additional stents in the first group. Thus, the procedure time was significantly higher in the first group. Nevertheless, stent diameters and procedure success rates were similar in both groups.
Conclusions
Although stenting of the RVOT in ToF and variants provides a safe and effective palliative strategy, this procedure has a short-term benefit with only one procedure until corrective surgery. This study demonstrates that long-term treatment with propranolol given after RVOT stenting in ToF and variants, particularly under 3 months of age, may reduce the need for further interventions prior to complete repair.








