Introduction
Endometrial carcinoma is now considered a common female gynecologic cancer with increasing incidence in developed countries. It usually has good prognosis but 13–25% of patients are still liable to recurrence and metastasis [1, 2], which needs further studies for detecting novel targets and new therapies.
Cancer invasion and metastasis is responsible for poor patients’ prognosis [3], and epithelial-mesenchymal transition (EMT) has vital roles in such processes [4].
Epithelial-mesenchymal transition is the biological process by which epi-thelial cells lose their normal criteria and acquire mesenchymal properties, thus enabling them to invade and metastasize [5], promoting cancer progression and spread [6].
The tyrosine kinase receptor which is known as recepteur d’origine nantais (RON), discovered in 1993, has been found to be overexpressed in many cancers [7, 8]. RON overexpression was associated with metastasis and poor prognosis [9]. Epithelial-mesenchymal transition, which explained invasion, metastases and progression of many cancers, was found to be mediated by several mediators as RON, ROR1 and SUSD2 (sushi domain containing 2) [10–12].
RON aberrant expression induces EMT in tumor cells, thus allowing migration, invasion and metastases [10]. So, targeting RON could be a promising cancer targeted therapy.
Although the role of RON in cancer progression has been studied in many cancers, its prognostic roles in endometrial adenocarcinoma have not been sufficiently clarified.
The recently detected Wnt receptor, ROR1 which is a tyrosine kinase-like orphan receptor, plays critical roles in embryogenesis. Aberrant ROR1 expression has been found in many cancers [11, 13]. ROR1 has various oncogenic roles in cancers [14].
It plays an oncogenic role in many tumors by increasing tumor proliferation, stimulating stemness [15], and activating EMT [12].
SUSD2 was primarily detected in mouse as a tumor-reversing gene.
Human SUSD2 is located on chromosome 22 and encodes an 822-mino acid type I membrane protein containing Sushi domains, which play important roles in adhesions between cells and cell-matrix adhesion [16]. Recently, dysregulated expression of SUSD2 was found in many cancers and associated with cancer progression, pointing to its oncosuppressive roles in cancers.
The aim of this study was to evaluate tissue expression of RON, ROR1 and SUSD2 in endometrial carcinoma and atypical endometrial hyperplasia using immunohistochemistry and correlate their expression with clinical, pathological and prognostic parameters of patients.
Material and methods
In the present study we included samples from 100 patients with endometrial carcinoma.
Cases were primarily collected by endometrial biopsy in the Gynecology and Obstetrics Department diagnosed in the Pathology Department. Cases underwent the ope-rations of total abdominal hysterectomy and bilateral salpingo-oophorectomy with or without lymphadenectomy in the Gynecology and Obstetrics Department and General Surgery Department, Faculty of Medicine, Zagazig University Hospitals. All surgically operated samples were sent to the Pathology Department for final diagnosis, grading and staging.
Patients were sent to the Medical Oncology Department and to Clinical Oncology and Nuclear Medicine for further management and follow-up for survival and recurrence.
We included 100 tissue blocks containing samples from patients with endometrial carcinoma and adjacent tissues of atypical endometrial hyperplasia.
Exclusion criteria
Cases with insufficient material in paraffin blocks and cases diagnosed with other endometrial malignancies such as endometrial stromal sarcoma and leiomyosarcoma were excluded.
Immunohistochemistry
For immunohistochemistry sections were incubated with primary anti-RON (1 : 500; ab52927; Abcam, Cambridge, Cambridgeshire, UK), anti-ROR1 (1 : 50, #564464, BD Biosciences, USA) and anti-SUSD2 antibodies (Rabbit polyclonal, Sigma) using the Leica Bond RX system (Leica Microsystems, USA) at a dilution of 1 : 100 at 4°C overnight.
Evaluation of RON, ROR1 and SUSD2 expression in stained tissues
The stained sections were evaluated by assessment of staining intensity and staining percentage. The staining intensity was divided into 0 (no stain), 1 (weak stain), 2 (moderate stain), and 3 (strong stain).
The staining distribution percentages were 0 (no staining ), 1 (1–25%), 2 (25–50%), and 3 (50–100%).
Multiplying scores of intensity and percentage yields final stain scores from 0 to 12. We considered a score of 4 as a cut point below which scores are considered low and above which scores are considered high to facilitate statistical analysis.
Statistical analysis
All statistics were performed using SPSS Statistics 22.0 for Windows (IBM Corp.). Continuous variables were expressed as the mean ±SD and median (range), and the categorical variables were expressed as a number (percentage). Percentages of categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test as appropriate.
Recurrence free-survival (RFS) was calculated as the time from surgery to date of recurrence or the most recent follow-up contact that the patient was known to be recurrence free. Progression-free survival (PFS) was calculated as the time from start of treatment to the date of disease progression or the most recent follow-up contact that the patient was known to be progression free. Overall survival (OS) was calculated as the time from diagnosis to death or the most recent follow-up contact (censored). Stratification of RFS, PFS, distant metastasis-free survival (DMFS), and OS rates were estimated using a Kaplan-Meier plot, and compared using the log-rank test. All tests were two sided. A p-value < 0.05 was considered significant.
Results
Table 1 shows that upregulation of RON and ROR1 and down regulation of SUSD2 expression were found in endometrial carcinoma more than atypical endometrial hyperplasia (p < 0.001) (Fig. 1).
Table 1
Association between RON, ROR1 and SUSD2 expression and histopathology
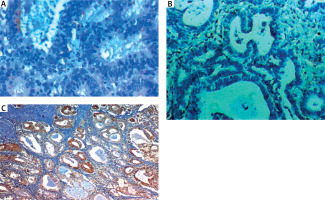
Fig. 1
Low RON expression in endometrial hyperplasia (A), low ROR1 expression in endometrial hyperplasia (B), high SUSD2 expression in endometrial hyperplasia (C)
For RON expression in endometrial carcinoma and the association with clinicopathological parameters see Table 2 and Figure 2.
Table 2
Clinicopathological and follow-up parameters, RON, ROR1 and SUSD2 expression and outcome of 100 patients with endometrial carcinoma
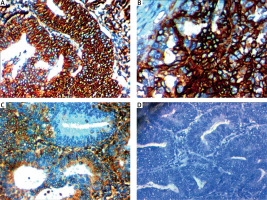
Fig. 2
RON expression in endometrial carcinoma (EC). High RON expression in serous EC high grade stage III 400× (A), high RON expression in endometroid EC high grade stage III 400× (B), low RON expression in endometroid EC low grade stage II 400× (C), negative RON expression in endometroid EC low grade stage I 400× (D)
High RON expression was significantly associated with large tumor size, high grade, presence of cervical stromal invasion, presence of adnexal invasion, presence of lympho-vascular invasion (p < 0.001), extent of myometrial invasion (p = 0.006), parametrial invasion (p = 0.008), serosal invasion (p = 0.005), positive peritoneal cytology (p = 0.004), presence of lymph node metastases (p = 0.003), distant metastases (p = 0.009), and advanced Internatio-nal Federation of Gynecology and Obstetrics (FIGO) stage (p = 0.002).
No significant association was found between RON expression and age of patients (Table 3).
Table 3
Association between RON, ROR1 and SUSD2 expression and clinicopathological features in endometrial cancer patients (N = 100)
For RON expression in endometrial carcinoma and the association with prognostic and follow-up parameters see Tables 4 and 5 and Figures 5 and 6.
Table 4
Association between RON, ROR1 and SUSD2 expression and patients’ outcome in endometrial cancer patients (N = 100)
Table 5
Association between RON, ROR1 and SUSD2 expression and survival rates in endometrial cancer patients (N = 100)
High RON expression was significantly associated with poor response to therapy (p = 0.046), higher incidence of disease progression (p = 0.018) and higher incidence of tumor recurrence (p = 0.004).
High RON expression was significantly associated with lower RFS rate (p = 0.002), PFS rate (p = 0.008), DMFS rate (p = 0.019) and OS rate (p < 0.001).
For ROR1 expression in endometrial carcinoma and association with clinicopathological parameters see Table 2 and Figure 3.
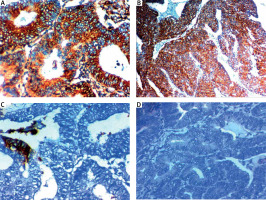
Fig. 3
ROR1 expression in endometrial carcinoma (EC). High ROR1 expression in serous EC high grade stage III 400× (A), high ROR1 expression in endometroid EC high grade stage III 400× (B), low ROR1 expression in endometroid EC low grade stage II 400× (C), negative ROR1 expression in endometroid EC low grade stage I 400× (D)
High ROR1 expression was significantly associated with older age of the patient (p = 0.031), large tumor size (p = 0.003), high grade (p = 0.007), presence of cervical stromal invasion, presence of adnexal invasion, extent of myometrial invasion, presence of lymph node metastases, positive peritoneal cytology, advanced FIGO stage (p < 0.001), presence of lympho-vascular invasion (p = 0.003), parametrial invasion (p = 0.004), serosal invasion (p = 0.004) and presence of distant metastases (p = 0.029).
For ROR1 expression in endometrial carcinoma and the association with prognostic and follow-up parameters see Tables 4 and 5 and Figures 5 and 6.
High RON expression was significantly associated with higher incidence of disease progression (p = 0.044) and unfavorable survival (p < 0.001).
No significant association was found between ROR1 expression and poor response to therapy or tumor recurrence.
High ROR1 expression was significantly associated with lower RFS rate (p = 0.032), PFS rate (p = 0.013), DMFS rate (p = 0.019) and OS rate (p < 0.001).
For SUSD2 expression in endometrial carcinoma and the association with clinicopathological parameters see Table 2 and Figure 4.
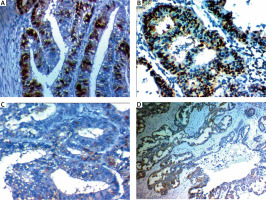
Fig. 4
SUSD2 expression in endometrial carcinoma (EC). High SUSD2 expression in endometroid EC low grade stage I 400× (A), high SUSD2 expression in endometroid EC low grade stage II 400× (B), low SUSD2 expression in serous EC high grade stage III 200× (C), low SUSD2 expression in serous EC high grade stage III 400 × (D)
Low SUSD2 expression was significantly associated with older patient age (p = 0.002), large tumor size (p = 0.003), high grade (p = 0.005), presence of adnexal invasion (p = 0.023), presence of lympho-vascular invasion (p = 0.021), extent of myometrial invasion (p = 0.002), serosal invasion (p = 0.005), presence of lymph nodes metastases (p = 0.003), distant metastases (p = 0.023), and advanced FIGO stage (p = 0.011).
No significant association was found between SUSD2 expression and cervical stromal invasion, positive peritoneal cytology or parametrial invasion.
For SUSD2 expression in endometrial carcinoma and association with prognostic and follow-up parameters see Tables 4 and 5 and Figures 5 and 6.
Fig. 5
Five-year disease-free survival (DFS) rate of included patients with endometrial carcinoma (A), DFS rate in association with RON expression (B), DFS rate in association with RON and ROR1 expression (C), DFS rate in association with SUSD2 expression (D)
DFS – disease-free survival

Fig. 6
Five-year overall survival (OS) rate of included patients with endometrial carcinoma (A), OS rate in association with RON expression (B), OS rate in association with RON and ROR1 expression (C), OS rate in association with SUSD2 expression (D)
OS – overall survival
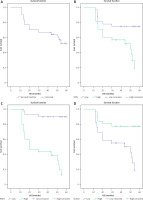
Low SUSD2 expression was significantly associated with poor response to therapy (p = 0.005), higher incidence of disease progression (p = 0.021) and higher incidence of tumor recurrence (p = 0.023).
Low SUSD2 expression was significantly associated with lower RFS rate (p = 0.008), PFS rate (p = 0.023), and DMFS rate (p < 0.001).
Discussion
In the present study we assessed levels of RON at the protein level using immunohistochemistry in endometrial carcinoma and found that RON was overexpressed in tissues and it was associated with poor clinicopathological parameters and unfavorable outcomes. These results were similar to results obtained by Qin et al. [9] and Zhuang et al. [17].
Qin et al. [9] found that high RON expression markedly increased the proliferation, migration, and invasion of malignant cells, while its knockdown had the opposite effect; additionally, targeted therapy against RON, with the RON inhibitor BMS777607, antagonizes the effects of RON overexpression.
All these results collectively showed the role of RON in increasing proliferation and progression of endometrial adenocarcinoma.
Similar roles of RON were found in different types of cancers [18, 19].
The roles of RON in cancer invasion, spread and progression are mostly due to activation of EMT, the process that was incriminated in cancer progression.
RON activation was shown to induce spindle morphology in tumor cells, thus facilitating invasion and spread [20].
Moreover, RON overexpression was found to affect many EMT biomarkers such as E-cadherin, which is a marker of intercellular adhesions in epithelial cells, and vimentin [21]. Moreover, RON expression allows endometrial carcinoma progression and promotes deep invasion into the myometrium [22].
RON also controls vimentin expression in endometrial carcinoma tissues; previous studies showed that increased vimentin expression promotes the migration and invasion of tumor cells [23].
So, RON played roles in facilitating cancer invasion and progression through induction of EMT of endometrial adenocarcinoma.
Additionally, RON could control EMT through several pathways, such as RAS-MAPK and PI-3K-Akt pathways [24], and SMAD and JAK pathways [25, 26].
Based on our results and results of previous studies, RON could regulate cell proliferation and EMT, which allow invasion and metastases in endometrial cancer (EC) cells, which promote the development of EC.
For further confirmation of the role of RON in progression of endometrial carcinoma we assessed levels of 2 other incriminated markers of EMT in endometrial carcinoma: ROR1 andSUSD2.
We found that ROR1 was overexpressed in endometrial carcinoma tissues and its expression was positively associated with progression and unfavorable patients’ outcome.
Similar results were reported by Liu et al. [14] and Henry et al. [27], who confirmed the role of ROR1 as a novel prognostic marker and therapeutic target in EC.
We observed that patients with high expression of ROR1 had lower OS and PFS rates in comparison with patients with low ROR1 expression levels.
We found an association between ROR1 expression and higher grade and advanced stage of endometrial carcinoma, which was similar to the results of Liu et al. [14] and those of Zhang et al. [28] in ovarian and pancreatic cancer.
ROR1 was found to play a role in cancer progression through activation of EMT through activation of Wnt signaling [29, 30], which was proved to be associated with metastasis in ovarian cancer [31].
Previous studies showed that ROR1 played an important role in the EMT process, which has a critical role in controlling cancer metastases [32, 33].
Regarding the association between ROR1 expression and survival rates, it was found that ROR1 overexpression was related to unfavorable survival rates and it could be considered a promising novel therapeutic target for recent management of EC.
The recently detected ROR1 inhibitor cirmtuzumab is a monoclonal antibody that has proven to be effective in inhibiting ROR1 signaling in ovarian cancer and chronic lymphocytic leukemia (CLL) [34, 35].
Another ROR1-targeting therapy which is called ROR1 chimeric antigen receptor T cell therapy, as described by Berger et al. [36], was tried as targeted therapy in CLL and triple negative breast carcinoma.
These new ROR1 inhibitors and targeted therapies could be beneficial to EC patients, particularly those with high expression of ROR1.
Our study confirms the prognostic role of ROR1 in endometrial carcinoma progression and points to the future benefits of ROR1-targeting therapies in EC patients.
We observed a positive association between RON and ROR1 expression in tissues of endometrial carcinoma; both biomarkers performed their work through activation of EMT and both are associated with cancer progression and unfavorable outcomes.
We assessed expression of another marker, SUSD2, which has variable prognostic roles in different cancer types.
Several studies have assessed associations between SUSD2 expression and prognosis of many cancers, e.g. breast, colon [16, 37], but its role in endometrial carcinoma has not been clarified yet.
Our study demonstrated that tissue protein expression of SUSD2 was downregulated in endometrial carcinoma tissues, its low expression was related to unfavorable prognostic and clinicopathological parameters and its expression was inversely associated with RON and ROR1 expression in tissues of endometrial carcinoma.
Similar results were obtained in hepatocellular carcinoma tissues in the study of Liu et al. [38], which showed that SUSD2 expression was reduced in malignant tissues more than adjacent non-neoplastic tissues, suggesting that SUSD2 down-regulation plays important roles in carcinogenesis and cancer progression.
A possible explanation of our results is that SUSD2 downregulation led to an increased rate of cell proliferation and reduced apoptosis; thus, SUSD2 had a tumor suppressor role and inhibited tumor growth [38].
Additionally we observed that high levels of SUSD2 markedly reduced invasiveness and migration ability of endometrial carcinoma cells, and we found that it was inversely associated with the EMT markers ROR1 and RON1.
Moreover, we found that high levels of SUSD2 expression in the tumor were associated with improved patient survival, which points to the tumor suppressor role of SUSD2. Similar results were obtained in serous ovarian carcinoma by Sheets et al. [39, 40], who reported that high SUSD2 expression could inhibit ovarian carcinoma metastasis.
Different results from ours were obtained in some studies on different cancer types.
Zhang et al. [41] reported that SUSD2 expression is upregulated in EC cells, associated with unfavorable prognosis, and its downregulation improves patients’ prognosis. Additionally they concluded that SUSD2 could be considered a chemotherapeutic target in EC. Their explanation for their results was that SUSD2 participates in apoptosis and cell senescence in EC cells and thus affects invasiveness and spread.
Also the results of Xu et al. [42] showed that high expression of SUSD2 increased ovarian cancer metastasis.
Those contrasting results could be explained by differences in methods of tissue expression, subcellular localization of SUSD2 and different metastasis mechanisms [43, 44]. Also the results of Watson et al. [16] showed that high SUSD2 expression in breast cancer enhanced cancer cell invasion and metastases.
Conclusions
We conclude that upregulation of RON and ROR1 in addition to downregulation of SUSD2 might be considered a promoting factor for EC cells’ proliferation, migration, EMT, and invasion.
Recommendations
We recommend performing further studies on gene expression of studied markers, aiming at their appraisal as novel therapeutic targets for treatment of endometrial adenocarcinoma.
Moreover, we recommend assessing markers’ expression in different variants of endometrial carcinoma in the recent molecular classification of endometrial cancer, to detect their roles.
Pre-operative detection of glandular cells (GC) in cervical smear analysis might be a predictor for endometrial cancer local recurrence [45]. Relative telomere length (RTL) in cell-free DNA (cfDNA) for EC, and cfDNA RTL analysis might be a potential diagnostic tool for early EC detection, progression, staging, and grading.
However, further studies are needed to confirm these results focusing on high-risk patients who might benefit from this tool, as TL shortening is not specific for EC [46].
We recommend investigating correlations between studied markers’ expression, pre-operative detection of GC in cervical-smear analysis and cfDNA RTL analysis for early detection of endometrial cancer recurrence.








