Summary
Radial artery constriction in response to the puncture is a very common phenomenon. It is gradual and does not always indicate an upcoming fully symptomatic spasm while passing through the artery. Unsuccessful first puncture attempt and female sex are risk factors for radial spasm. A hydrophilic-coated sheath can be used to decrease the risk of spasm. Angiographic narrowing at the tip of the sheath predicts a symptomatic spasm. To avoid a radial spasm, the puncture should be succeeded at the first attempt. The spasm can be overcome by a coronary guidewire or a hydrophilic catheter.
Introduction
Nowadays, the radial artery is recommended as the standard access for coronary interventions and is used in more than 90% of cases at selected centers [1]. It is associated with the lowest risk of complications [2] as well as better convenience for the patient [3]. However, sometimes a conversion to a different arterial access is required. This is usually caused by a failed attempt to puncture the artery, winding course of the radial or brachial artery or radial artery spasm [4]. A radial artery spasm can be defined as a sudden, temporary narrowing of the radial artery due to vasoconstriction. It can be diagnosed clinically, based on sudden difficulty in advancement of a guidewire or catheter, or angiographically. At several catheterization laboratories (CathLabs), a combination of vasodilating and analgesic medications, often referred to as a radial cocktail, are administered to the artery to reduce the risk of spasm. The applied medications include, among others, nitroglycerin, verapamil [5] or lidocaine to prevent spasm, as well as heparin to prevent thrombosis [6]. Radial artery spasm interrupts and prolongs the procedure. In addition, it causes unnecessary pain for the patient. The delay is especially unfavorable in patients with ST-elevation myocardial infarction or hemodynamic instability [1] and can be potentially fatal. Currently, many factors concerning radial artery spasm have been confirmed, however, the reported results significantly vary between studies [7–10]. In addition, evidence on spasm prevention is also heterogeneous [10, 11], and the described methods of management are limited. This study was aimed at identifying factors increasing the risk of the spasm, and determining ways to avoid such a situation.
Aim
This study was aimed to present preliminary, short-term results regarding radial artery spasms of the first 103 consecutive patients enrolled in the Radial Patency observational study.
Material and methods
Patients were included from 2022 to 2023 at a tertiary referral hospital with a catheterization laboratory. The Radial Patency study was designed to observe factors contributing to long-term loss of patency in patients after coronary procedures via radial access. The inclusion criterion was undergoing an invasive coronary procedure via radial access. The exclusion criterion was conversion to a different arterial access. Detailed procedure characteristics associated with the puncture site and technique were collected. In cases where it was possible without potential harm to the patient, radial artery angiography was performed before and after the procedure (70 cases). Main exclusion criteria for the angiography were immediate urgency to perform an angioplasty, advanced renal failure and high contrast administration during the procedure. Radial spasm was assessed subjectively by the operator. It was defined as significant resistance while passing the radial artery with a guidewire or catheter. Angiography was used to differentiate spasm from tortuosity in case of guidewire passing difficulty. Based on the operator’s decision, 79 out of 103 patients received an intraarterial radial cocktail according to their clinical state. The radial cocktail consisted of 300 μg of nitroglycerin and 40 mg of lidocaine. In some cases, the operator preferred administering nitroglycerin only (200–300 μg). The study was approved by the author’s institution Ethical Committee (approval no. 1072/6120.260.2022, 16 Nov. 2022) and performed in accordance with ethical standards indicated in the Declaration of Helsinki.
Statistical analysis
Categorical data were described as counts and percentages, and assessed by the χ2 test. Investigated risk factors were presented as odds ratio with a 95% interval and assessed by the χ2 test or Fisher’s exact test for expected counts < 5. Distribution of the data was assessed using the Shapiro-Wilk test. Data following normal distribution were compared using the Student’s t-test and presented as means with standard deviation. If variance was unequal according to the Brown-Forsythe test, Welch’s t-test was used instead. Data following distribution different from normal were compared using the Mann-Whitney U test and presented as medians with quartiles. The authors abstained from multivariate investigation as the number of events was too low to create a reliable regression model. The level of statistical significance was set as p < 0.05. All statistical analyses were carried out using JASP, version 0.17.1 (University of Amsterdam, the Netherlands).
Results
The study included 103 patients, among which 70 underwent radial angiography, while others were disqualified due to comorbidities contraindicating greater contrast administration, complexity of the procedure or the urgency to perform immediate percutaneous coronary intervention (PCI). The most common indication for the procedure was chronic coronary syndrome (45.9%), followed by non-ST segment elevation myocardial infarction (NSTEMI) (18.4%), ST-segment elevation myocardial infarction (STEMI) (10.2%), unstable angina (9.2%) and others (Table I). The majority of patients were male. The median age was 67 (60.74) years. The vast majority of patients suffered from hypertension, which was followed by hyperlipidemia as the second most common comorbidity (approximately half of the patients). Detailed patient characteristics are presented in Table I.
Table I
Investigated characteristics regarding patients and procedures
[i] BMI – body mass index, LVEF – left ventricular ejection fraction, WBC – white blood cell count, RBC – red blood cell count, Hb – hemoglobin, INR – international normalized ratio, APTT – activated partial thromboplastin clotting time. Continuous data are presented as mean (SD) median (Q1–Q3) according to distribution. Categorical data are presented as counts with percentages in brackets. Presented p-values regard significant difference tests.
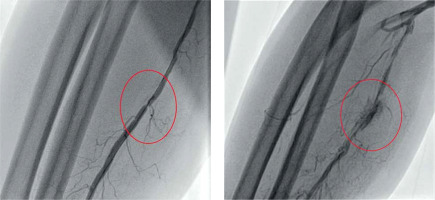
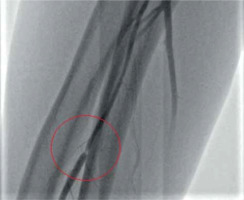
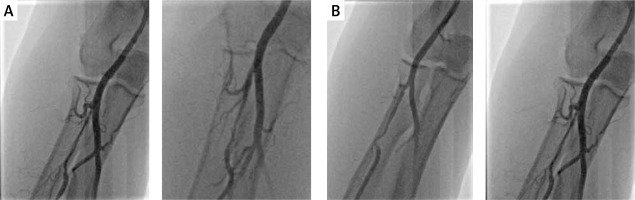
The gross majority of the patients was hemodynamically stable (Killip class I). The incidence of reported radial spasm was 24.3% (25 patients). The preferred side for radial access was the right-hand side according to the local protocol (nearly 90% of procedures). The most common type of the procedure was coronary angiography alone. In each case, the outer size of applied sheath was 6F. No puncture was performed under ultrasound guidance as it is treated as an optional bailout in the local protocol and, according to the operator, palpable guidance was sufficient in all instances. Standard radial access was applied in all of the cases; the distal radial access was not used. Details of the procedures are demonstrated in Table II. In one of the cases, initial angiography allowed the operator to choose coronary guidewire – Hi-Torque Balanced Heavyweight (Abbott Cardiovascular, Plymouth, MN, US) as the first choice to cross a narrow spasm in the radial artery, which allowed for uninterrupted guidewire and catheter delivery (Figure 1 A). In another case, a coronary guidewire – Hi-Torque Balanced Middleweight (Abbott Cardiovascular, Plymouth, MN, US) was used as a successful bailout after initial failure with a standard wire (Figure 1 B). In 2 other cases, a hydrophilic-coated Sheathless Eaucath catheter (Asahi Intecc, Seto, Japan) was used as a bailout after spasm involving a standard catheter – in this case to perform an angiography, and in the other, to perform coronary angioplasty. Two procedures involved spasm in a patient presenting with STEMI. In the initial angiography, there was a narrow focal occlusion (Figure 2), which had to be forced due to the urgent need to perform the PCI. In both cases, the post-procedural radial angiography revealed a major dissection in the place of previous focal narrowing (Figure 2).
Table II
General procedure and access-site characteristics
[i] STEMI – ST-elevation myocardial infarction, NSTEMI – non ST-elevation myocardial infarction, UA – unstable angina, CCS – chronic coronary syndrome, FFR – fractional flow reserve, OCT – optical coherence tomography, IVUS – intravascular ultrasound. Continuous data are presented as median (Q1–Q3) according to distribution. Categorical data are presented as counts with percentages in brackets.
Figure 1
Spasms overcome by application of a coronary guidewire. A – Spasm crossed with Hi-Torque Balanced Heavyweight (Abbott Cardiovascular, Plymouth, MN, US) – angiography before and after the intervention. B – Spasm crossed with Hi-Torque Balanced Middleweight (Abbott Cardiovascular, Plymouth, MN, US) – angiography before and after the intervention
Radial artery spasm was more common in women than in men (OR = 2.94). Any other characteristics of the patients, such as height, weight, body mass index (BMI) or any comorbidities were not observed to make a significant difference in the spasm occurrence. A detailed list of all the compared characteristics is presented in Table I. Amongst procedural factors, failure of the first attempt to puncture the artery significantly increased the odds for a spasm. The administration of a radial cocktail was not found to be effective in spasm prevention. The average artery diameter at the tip of the sheath in the initial angiography was significantly smaller among the patients who later experienced a spasm. This can be identified as a focal spasm which was demonstrated in Figure 3. Such situation is probably due to the sheath forcibly dilating the spasm. No spasms were observed in procedures with the use of a hydrophilic sheath and it has been found to be a protective factor against radial spasm (hydrophilic vs. non-hydrophilic OR = 0.10, p = 0.036). There was no significant association between operator’s experience and spasm occurrence. Other explored factors were not statistically significant. A detailed comparison of procedural factors is presented in Table I.
Overall, the average maximal dimension of the radial artery was greater than the ulnar artery. There was a group of patients having the ulnar artery substantially greater than the radial one – 18.5% – but most commonly, the radial artery was dominant (43.1%) or the two arteries were comparable in size (38.5%).
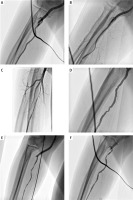
Radial artery angiographic image indicated at least a mild sign of a spasm in a substantial group of patients (37.1%). However, this did not always result in a symptomatic spasm interrupting the procedure. It also was not a significant risk factor for spasm. The occurrence of spasmatic image at the end of the procedure was greater – 60.1%. Detailed results of artery dimension measurements are presented in Table III. As demonstrated in Figure 4, spasms in the radial artery had diverse morphology. Two main determined morphologies were focal or segmental. The location also varied – the spasm could be limited to a single part of the artery or involve the whole vessel. The incidence of different types is presented in Table III.
Table III
Angiographic findings
Figure 4
Presentation of different spasm morphology and location. A – reference image, B – segmental spasm at the tip of the sheath, C – long segmental spasm in cubital fossa and below, D – focal spasm, E – generalized segmental spasm, F – disseminated focal spasm
The median of arterial blood pressure measured in the radial artery at the beginning of the procedure was 151/71 (132/60; 180/80) mm Hg. The administration of a radial cocktail resulted in a decrease in systolic pressure by 30 mm Hg, diastolic pressure by 10 mm Hg and mean arterial pressure by 14 mm Hg (presented as medians). The occurrence of hypotension after radial cocktail administration was rare (5.7%). The median of arterial blood pressure at the end of the procedure was 139/70 (125/60; 150/80) mm Hg.
Discussion
The main findings of the current study are the following risk factors for radial artery spasm: female sex, multiple puncture attempts and angiographical small artery diameter at the tip of the sheath, while a hydrophilic-coated sheath is a protective factor. The study also allows to note that a hydrophilic-coated catheter or coronary guidewire can be applied to overcome the spasm. In addition, the study revealed that most often, the radial artery is the dominant (43.1%) or co-dominant (38.5%) one in the forearm, further justifying its position as the preferred access in this location. However, the size of the radial artery varies between patients, and is significantly smaller than that of the ulnar artery (18.5%). Other risk factors are still worth further investigation, especially the application of a radial cocktail on a group that will allow for multivariate analysis.
The observed incidence of spasm was relatively high; this is probably due to operator’s subjective assessment of a spasm, defined here as an increased resistance while passing a device via the artery. Radial artery spasm remains a common and significant problem in interventional cardiology. It can cause unnecessary pain to the patient and prolong the procedure, as well as lead to a major radial dissection observed in this study.
In our research, administration of the radial cocktail was not found to significantly decrease the incidence of spasm. However, this could be due to selection bias, as it was administered based on the operator’s decision. Similarly, Curtis et al. did not observe nitrates to cause significant differences in radial artery spasm prevention. The other investigated pharmacological prophylaxis – midazolam, fentanyl – was inefficient as well. Lignocaine was not investigated [10]. Nonetheless, in a different study conducted by Gopalakrishnan et al. [12], the authors stated that diazepam administration prior to the procedure decreased the incidence of radial spasm. In addition, they claimed that topical nitroglycerin administration was also protective.
However, the study carried out by Chen et al. is discordant with our trial, as well as the aforementioned study. Chen et al. reported that intraarterial nitroglycerin demonstrated a protective effect against radial artery spasm compared to heparin only [11]. This is one of the older studies on the subject and based on these results, nitroglycerin is often administered in the radial cocktail to prevent spasm. To the contrary, a recent systematic review by Abdelazeem et al. denied the efficacy of both intraarterial and topical nitroglycerin in spasm prevention. Nevertheless, it was stated that subcutaneous nitroglycerin administration is an effective method of prevention [13].
Female sex was observed in our study as a significant risk factor for spasm. This is a commonly reported relationship. Rathore et al. reported it, among others, as an independent risk factor [7]. In a study conducted by Jia et al., it was also confirmed that female sex was an independent predictor for radial artery spasm [8]. Similar results were reported by Curtis et al. [10] The probable cause of female sex being a significant risk factor is the difference in body size, and artery size in particular. Smaller arteries are more prone to spasm as there is a greater risk of artery irritation, especially in the case of sheath oversize [14]. In addition, anxiety associated with the procedure is more common and more pronounced amongst women, which also increases the risk of spasm development [15].
Other risk factors, including diabetes, smoking, and younger age, not observed to be significant in our research, were however reported by some of the studies [7, 10].
Multiple puncture attempts were identified as a risk factor in our research. This was also confirmed by Jia et al. [8]. As failure of the first puncture attempt was determined to be a risk factor for radial artery spasm, it is important to improve first-attempt success rate. According to the RAUST trial conducted by Seto et al., ultrasound assistance to obtain access improved the success rate. In addition, it also decreased the average time to obtain the arterial access [16]. Therefore, ultrasound guidance seems to be beneficial in all patients, and especially in a group where the access is difficult to achieve. Such patients could be identified using the predictive score of radial artery spasm developed by Giannopoulos et al. [9]. Unfortunately, the score does not include sex, which was determined to be a risk factor in several of the aforementioned studies [7, 8, 10]. According to the meta-analysis conducted by Fernandez et al., ulnar access is comparable to radial access in terms of its safety and efficacy [17]. As observed in our study, the disproportion in the forearm artery is varying between the patients, with an incidental ulnar dominance. Therefore, ulnar access seems like a reasonable alternative to radial access, which could be considered especially in patients with a radial artery smaller than that ulnar one. This could be determined before in ultrasound, which could be further used to facilitate the puncture.
Hydrophilic-coated devices were observed in our study to be both a means of prevention against spasm, as well as management. The previously mentioned research by Rathore et al. reported hydrophilic-coated sheath as an effective protective factor [7] – similarly to our results. An observational study carried out by Borrie et al. showed efficacy of the hydrophilic-coated Sheathless Eaucath catheter in overcoming radial artery spasm. The application of the catheter allowed to pass through every one of the 44 spasms [18]. Similarly, in a case reported by Chyrchel et al., the Sheathless catheter was used to overcome spasm in a patient requiring urgent revascularization due to myocardial infarction with left main artery closure [19].
Although signs of spasm in initial angiography do not always indicate an intraprocedural spasm, they can be useful in selected cases. First of all, tight narrowing at the tip of the sheath was found to be a predictor of radial spasm. In addition, if the overall image of the artery shows signs of a severe spasm and the artery is substantially tortuous, the operator can also suspect a difficulty in passing the artery. Therefore, it could be beneficial to run a scout fluoroscopy to assess the artery in a selected group of patients who have the risk factors for coronary spasm. Such image could guide the operator to consider alternative approaches as the first choice, including coronary guidewire or hydrophilic catheter to avoid unnecessary pain for the patient and damage to the artery intima.
As reported by Ying et al., flow-mediated dilation can be used as a feasible non-pharmacological method to relieve spasm. In their study, application of this technique was comparable to nitroglycerin administration and guaranteed a significantly faster recovery compared to the control, while not causing adverse effects [20].
This study comes with certain limitations. Multivariate analysis was not performed due to the small number of cases. Therefore, this study should be treated as one generating a thesis, and the results should be confirmed on a larger group with multivariate analysis. Spasm occurrence was subjectively assessed by the operator performing the procedure. The measured artery dimensions are not meant to represent the resting anatomical size, rather the dynamics of the artery dimensions during the procedure, which are influenced by the sheath dilating the artery, spasm and other factors. Radial cocktail was administered according to the operator’s decision, therefore it might be biased towards the patients with a greater risk of spasm.
Conclusions
Current analysis demonstrates that risk factors for radial artery spasm include non-hydrophilic sheath coating, female sex, narrowing at the tip of the sheath and multiple puncture attempts. Gentle insertion of the sheath is advised as in a case of a developing spasm, forcible dilation of the vessel further traumatizes the artery and can possibly exacerbate it. The same risk factors have been reported by other researchers, with the exception of the narrowing at the tip of the sheath, which to our knowledge, has not been researched before. The radial artery was dominant or equivalent to the ulnar one in the majority of patients. Further research on other factors of radial spasm is required. Radial artery spasm can be managed by application of a coronary guidewire or hydrophilic-coated catheter.