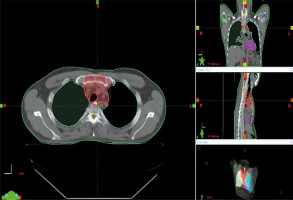
Introduction
Radiation therapy (RT) holds considerable significance in the treatment of lymphoma, thymoma, and thymic carcinoma, given their notable sensitivity to this modality [1–3]. While these malignancies are commonly known as the prevailing neoplasms affecting the anterior mediastinal compartment, their rarity and indolent natural history have posed challenges in establishing definitive guidelines for RT within the multidisciplinary treatment approach for thymic neoplasms. Furthermore, the rapid advancements in RT delivery techniques have added complexity to the evaluation of the potential benefits of current treatment practices, as historical data collected over several decades may not accurately reflect the outcomes. Notably, there is a paucity of randomized prospective clinical trials investigating the role of adjuvant RT following complete resection of thymoma or thymic carcinoma. Nevertheless, retrospective analyses utilizing the Surveillance, Epidemiology, and End Results (SEER) database, along with multiple small case series, have shed light on the potential benefits of adjuvant RT for specific subpopulations of patients with thymoma and thymic carcinoma. In the management of early-stage Hodgkin lymphoma, RT continues to be a crucial component of combined modality therapy, although concerns have arisen regarding late toxicity, which is dependent on the irradiated volume and radiation dose [1, 2, 4–10].
Radiation therapy for lymphoma
RT remains the preferred treatment modality in combination with chemotherapy for lymphoma. Gradually, the efficacy of radiation doses lower than the conventional 36–40 Gy has been demonstrated [3, 11, 12]. Lower radiation doses are expected to result in a decrease in late toxicity, as the incidence of toxicity is correlated with the radiation dose administered [13, 14]. Consequently, many centers and groups have already adopted lower doses. Furthermore, reducing the radiation fields should lead to a decrease in late toxicity by minimizing the irradiated volume of normal tissue, an assumption that recent data support [13, 14]. In cases where effective chemotherapy is utilized, involved-field radiotherapy (IFRT) has been shown to be sufficient [15]. However, the definition of IFRT has historically been ambiguous, often involving irradiation of lymph node regions based on the Ann Arbor staging diagram, which was not originally designed for defining radiation fields. A recent review of relapses in patients treated with chemotherapy alone revealed that most recurrences occurred in the initially involved lymph nodes [16].
With the advent of modern sophisticated techniques such as improved computed tomography (CT) scan imaging, fluorodeoxyglucose positron emission tomography (FDG-PET) scans, and more precise RT technology, it is now possible to customize radiotherapy for each patient, accurately delivering radiation to the initially involved volume while minimizing radiation exposure to normal tissues [17]. Involved-node radiotherapy (INRT), which encompasses solely the initially involved lymph node(s), can now replace IFRT, which includes the entire initially involved lymph node region [18–20]. INRT is being implemented in the EORTC-GELA randomized trial for patients with early-stage Hodgkin lymphoma. To achieve this level of precision, clear RT guidelines, adequate training of radiation oncologists, and the establishment of early retrospective or even prospective quality assurance programs are crucial [21, 22].
Guidelines
Involved-node radiotherapy
The patients should undergo an evaluation by the radiation oncologist prior to chemotherapy initiation. It is essential that all patients undergo pre- and post-chemotherapy cervical and thoracic CT scans. Whenever feasible, CT scans should be conducted in the treatment position. Similarly, the pre-chemotherapy PET-CT scan should also be performed in the same treatment position to aid in the identification of previously undetected involved lymph nodes. During the evaluation of CT scans, the presence of a radiologist is recommended. To ensure the accurate implementation of INRT, CT simulation, utilization of modern radiation therapy techniques (such as 3D-CRT, intensity-modulated radiation therapy (IMRT), or respiratory-gated radiotherapy), and the use of immobilization devices are strongly advised [23, 24].
Assessment of the response after chemotherapy should be carried out exclusively for each initially involved lymph node, utilizing CT scans. Complete response (CR) is defined as the complete disappearance of clinically and/or radiologically detectable disease. Partial response (PR) is characterized by a minimum of a 50% decrease in tumor size. An unconfirmed complete response (CRu) is defined as at least a 75% decrease in tumor size. Non-response (NR) is indicated by less than a 50% decrease or any increase in tumor size [25, 26].
Design of radiation fields
To ensure optimal quality during the delivery of radiotherapy (RT), it is recommended to conduct pre- and post-chemotherapy CT scans with patients positioned for treatment. The same principle applies to FDG-PET/CT scans. Additionally, fusion techniques are suggested to enable the alignment of pre- and post-chemotherapy CT scans. However, it is important to exercise caution when employing fusion techniques due to several underlying assumptions associated with the technique [27, 28].
Initially involved lymph nodes in CR or CRu
Cervical and axillary lymph nodes
A clinical target volume (CTV) is delineated to define the initial extent of the lymph node(s) before chemotherapy. The CTV encompasses the location of the involved lymph nodes and the extent of the disease. However, normal structures that have been displaced by enlarged lymph nodes, such as a neck muscle, are not incorporated into the irradiated volume. Whenever feasible, blood vessels are excluded from the CTV if the involved lymph nodes are clearly distant from them. In cases where a complete response with a visible lymph node remnant (CRu) is observed, the lymph node remnant should be included in the CTV. The planning target volume (PTV) is generated by adding a margin to the CTV, accounting for organ movement and set-up variations [29, 30].
Mediastinal area
A CTV is delineated to represent the initial volume of the mediastinal disease. In the case of a complete response CR, the CTV is limited to the boundaries of the normal mediastinum and should not extend beyond the lateral mediastinal boundaries. For CRu cases, the normal mediastinum is contoured and the CTV should not exceed the lateral mediastinal boundaries except where lymph node remnants are still present. It is advisable to avoid including blood vessels in the CTV, and to minimize lung toxicity, the length of the CTV should correspond to the length of the mediastinal mass or lymph node(s) before chemotherapy, while the width of the CTV should correspond to the width of the mediastinal mass or lymph node(s) after chemotherapy (Figure 1). The planning target volume (PTV) is generated by adding a margin to the CTV, considering organ movement and set-up variations [31, 32].
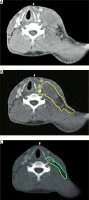
Figure 1
Clinical target volume (CTV) of a cervical tumor in complete response (CR) after chemotherapy. A – Pre-chemotherapy computed tomography (CT) scan. B – Contouring of the initial tumor volume (yellow color). C – CTV contouring takes into account the initial tumor volume on the postchemotherapy CT scan (green color)
Initially involved lymph nodes in PR
Cervical and axillary lymph nodes
In the context of partial remission of initially involved lymph nodes, the gross tumor volume (GTV) is defined as the lymph node remnant(s) alone. The clinical target volume (CTV) encompasses the initial volume of the lymph node(s) before chemotherapy. Two planning target volumes (PTVs) should be delineated. PTV1 comprises the CTV, including the GTV (i.e., initial tumor mass and lymph node remnant(s)), with an added margin to account for organ movement and set-up variations. PTV2 consists of the GTV alone, also with a margin to consider organ movement and set-up variations [33].
Mediastinal area
Considering that the initially involved lymph node(s) or tumor mass is (are) in partial remission, the first step is to delineate the GTV, which represents the remaining lymph node remnant(s) or the residual mass alone. The CTV is defined as the initial volume of the mediastinal mass. It is advisable to avoid including blood vessels and the heart whenever possible, in order to minimize lung toxicity. Two PTVs should be outlined: PTV1 encompasses the CTV along with the GTV, which includes the initial tumor mass and the lymph node remnant(s), while accounting for organ movement and set-up variations. PTV2 consists of the GTV alone, with a margin to accommodate organ movement and set-up variations [34, 35].
Treatment and dose prescription
The specification of radiation dose follows the recommendations of ICRU 50/62 [36]. The PTV should receive a dose ranging between 95% and 107% of the prescribed dose. Contouring and treatment planning using three-dimensional conformal radiation therapy (3D-CRT) are essential and should be employed (Figure 2). If the initially involved lymph nodes are more than 5 cm apart, separate fields should be utilized. The use of 3D-CRT, IMRT, or respiratory-gated RT is strongly recommended. The choice of the RT delivery technique ultimately rests with the physician. In the case of conventional RT, with anterior and posterior fields, the field size should be a minimum of 5 × 5 cm. RT should be administered with a fractionation scheme of five fractions per week, each delivering 1.8–2 Gy. Portal imaging of all fields should be conducted daily within the first 2 days of treatment and once a week thereafter. Daily portal controls are recommended as per the protocol. Additionally, monthly quality assurance meetings should be organized involving radiation oncologists participating in the randomized trials [37–39].
Radiotherapy for thymoma
Complete surgical resection is associated with a 7-year survival rate of 82%. In contrast, incomplete resection and biopsy alone are linked to survival rates of 71% and 26%, respectively. The primary treatment approach for thymoma and thymic carcinoma without unresectable/metastatic disease involves en-bloc resection of the tumor and adjacent involved structures. However, when complete surgical resection is not feasible, there is a potential role for both RT and chemoradiotherapy in order to prevent recurrence [40].
RT has demonstrated its efficacy in improving local control rates and overall survival. The role of RT includes: adjuvant therapy for stage I and II patients, neoadjuvant treatment with or without chemotherapy for medically operable locally advanced cases, definitive RT for unresectable patients with stages III/IVa disease, and palliative therapy for symptomatic metastatic sites [41–43].
Guidelines
Stage I patients who have undergone R0 resection are typically observed without further treatment. The role of RT for stage II patients is still a subject of debate, but it is recommended for patients with specific risk factors, such as pleural invasion (T2b disease), close surgical margin (< 1 mm), higher grade (WHO B3), and tumors with invasion of surrounding tissues after resection (R1). In patients with stage III/IVa disease who have undergone subtotal resection (R1/R2), adjuvant RT can help reduce the occurrence of local recurrence (LR).
A prospective randomized study conducted in China included 29 stage I thymoma patients who were randomized to either observation after surgery or RT. The study showed no significant difference in recurrence patterns and metastases between the two groups [41]. The majority of patients in this study were under 65 years old, and AP/PA with oblique wedge pair techniques were used for RT planning. For lymphocyte-predominant histology, a radiation dose of 50 Gy in 25 fractions was delivered, while epithelial/mixed variants were treated with 60 Gy in 30 fractions. The 10-year overall survival (OS) rate was 92% for the surgery alone group and 88% for patients treated with adjuvant RT.
The role of RT for stage II patients remains controversial, with some single institution series reporting a decrease in local control rates in patients treated with adjuvant RT compared to observation [42–44]. Another multi-institutional retrospective review of 103 cases investigated the role of postoperative radiation therapy in completely resected thymoma cases, with different stages represented (17% stage I, 59% stage II, and 24% stage III) [42]. None of the patients included in this study were treated with chemotherapy. The median RT dose was 40 Gy, with 51% of patients receiving involved field RT and 49% receiving treatment of the entire mediastinum. The median follow-up period was 9.3 years, and the 10-year OS rates were 81% for the entire cohort, 100% for stage I, 90% for stage II, and 48% for stage III. In-field recurrences were not observed, and 70% of recurrences occurred within the pleura. Pleural failures were observed in 38% of patients with pleural invasion at diagnosis, and no correlation was found between RT doses greater than or equal to 40 Gy and reduction in recurrences in patients with pathological pleural invasion.
A comparison of outcomes for stage I and II patients treated with adjuvant RT was conducted at the University of Pennsylvania, involving 167 patients [44]. Among the 70 patients with stage IIb, 23 received surgery ± RT with an RT dose of 45–55 Gy in 25–33 fractions. With a median follow-up of 70.3 months, the local recurrence rate was 1.4%, with 1 patient experiencing recurrence in each group. There was no difference in overall survival (OS), with a 5-year OS rate of 91%. An updated subset analysis focusing on high-risk stage II patients who underwent complete resection was conducted by Berman et al., involving 175 patients treated between 1990 and 2008 at the University of Pennsylvania [45]. Among these patients, 62 had complete resection, and 37 had high-risk features and were treated with a dose of 50.4 Gy. The median follow-up period was 52 months, and the overall LR rate was 3.2% (8.3% after surgery and 0% after RT, p = 0.15).
The evaluation of the role of adjuvant RT on OS and cause-specific survival (CSS) involved 1464 patients from the Surveillance, Epidemiology, and End Results (SEER) database [46], and the median follow-up was 41 months. The median OS for patients treated with surgery alone was 80 months, while those treated with adjuvant RT had a median OS of 97 months. The 10-year OS rate for the total cohort was 42% for patients treated with RT, 41% (p = 0.06) for those without RT, and the 10-year CSS rates were 72% and 76% (p = 0.85), respectively. For patients with incomplete resection, the 10-year OS rates were 63% with RT and 46% without RT (p = 0.4), while the CSS rates were 81% and 80% (p = 0.9), respectively. The addition of adjuvant RT was correlated with an improvement in the 10-year OS rate for stage II–III patients (41% with RT compared to 35% without RT, p = 0.002), but there was no difference in CSS.
A pooled data analysis from three institutions was conducted by Curran et al. [47] to examine the role of mediastinal radiation therapy after complete or incomplete resection in 103 patients treated between 1960 and 1985. The 5-year OS rates were 67% for stage I, 86% for stage II, and 69% for stage III, with a relapse-free survival of 100%, 58%, and 53% for each stage, respectively. For stage II–III patients who underwent R0 resection, LR occurred in 53% of patients without RT and 0% in patients treated with RT. The overall LR rate for the entire cohort was 28% without RT and 5% with RT.
However, it is important to note that there are currently no conclusive studies that support specific radiation dose techniques for thymic malignancies. In order to foster debates regarding the ideal radiation method in the management of thymic malignancies, long-term prospective studies utilizing image-guided radiotherapy and contemporary surgical procedures are needed [48].
Radiotherapy techniques for thymoma
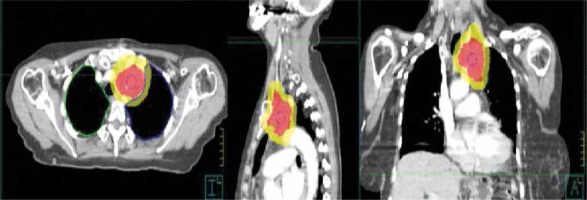
Advancements in RT techniques have contributed to a reduction in toxicity. The introduction of 3D-CRT compared to traditional two-dimensional techniques has allowed for the selection of optimal beam angles that deliver RT while minimizing the dose to critical structures [49]. The use of three-dimensional techniques is now considered a mandatory requirement for irradiation (Figure 3).
IMRT further optimizes the dose distribution to the tumor and normal tissues by delivering a non-uniform dose within the RT field, enhancing dosimetric conformity, and improving the therapeutic ratio. Proton therapy offers the advantage of delivering a sharp increase in dose at a specific depth within the tissue.
The implementation of four-dimensional (4D) treatment planning has been instrumental in measuring tumor motion and defining the internal target volume (ITV). Additionally, image-guided tools such as cone-beam CT (CBCT) have proven effective in reducing treatment volumes. In the pre-CT era, the target volume encompassed the gross tumor volume (GTV), bilateral mediastinal lymph nodes, and bilateral hilar nodes and/or mediastinum, excluding the hilum. However, in current practice, CTV is confined to the pre-surgical extent of the disease, including the entire surgical bed without elective nodal radiation, except for cases of thymic carcinoma. Postoperatively, a dose of 45–50.4 Gy, delivered in fractions of 180–200 cGy over 5–6 weeks, is typically administered with negative margins. For positive margins, a dose of 54 Gy is employed, while gross residual disease may require a higher dose of 60–70 Gy.
The emergence of adaptive RT techniques has allowed for treatment adjustments based on tumor response, breathing patterns, and changes in patient weight during the course of treatment. In some cases, stereotactic radiation therapy (SBRT) is used as a boost in patients with bulky, unresectable tumors.
Radiotherapy for thymic carcinoma
Thymic carcinoma is a relatively rare and aggressive type of thymic neoplasm compared to thymoma, with a 5-year survival rate of 35% [50]. Similar to thymomas, surgical resection is the primary treatment approach. However, the prognosis for thymic carcinoma is poor due to early metastases to various sites such as the pleura, lung, bone, brain, liver, as well as the mediastinal, cervical, and axillary lymph nodes [51, 52]. The role of RT in the multimodality treatment of thymic carcinoma, particularly in combination with specific chemotherapeutic agents, remains controversial.
Radiotherapy techniques
3D-CRT or IMRT techniques have led to increased conformality, allowing for dose escalation while reducing the dose to critical organs at risk (OAR). In the context of radiation therapy for thymic carcinoma, the OAR of concern are the heart, lungs, esophagus, and spinal cord. Specific dose constraints have been established for these organs to minimize potential toxicities. To mitigate pulmonary toxicity, it is recommended to restrict the V20 (volume receiving 20 Gy) to less than 30% and maintain a mean lung dose of less than 20 Gy, with particular attention to minimizing the V13 [53, 54], based on experiences from lung cancer studies. The entire heart has been assessed to tolerate a whole-heart dose of 35–40 Gy, with no more than one-third of the myocardium receiving 60 Gy. Whenever possible, it is recommended to keep the whole heart dose below 30 Gy [55]. The maximum dose to the spinal cord should remain below 45 Gy. Recent reports suggest a 5% to 10% incidence of radiation toxicity, possibly attributed to advancements in RT delivery techniques [56]. Death resulting from RT-related toxicity has been reported in 1% to 13% of cases [57].
Discussion
As chemotherapy has become more effective, IFRT has replaced RT with extended fields as the primary treatment approach for early stage unfavorable Hodgkin’s lymphoma, as supported by the German Hodgkin Study Group and the EORTC-GELA cooperative H8 trial [13, 15].
The EORTC-GELA radiotherapy group further reduced the radiation field size due to late toxicities, such as cardiovascular issues and second cancers, which were correlated with the size of the radiation fields. This led to the development of the concept of INRT, where only the initially involved lymph nodes are irradiated. Shahidi et al. recently evaluated this concept and found that recurrences typically occurred in the initially involved nodes in patients treated with chemotherapy alone [16].
During INRT, it is essential to irradiate the initial tumor volume. The decision to deliver radiation to the initial tumor volume was based on two main factors. First, it was considered an intermediate step between conventional involved-field irradiation and a more limited irradiation of the tumor remnants alone. Secondly, it was recognized that by delivering radiation exclusively to tumor remnants, no radiation would be delivered to lymph nodes in complete remission. The irradiation of lymph nodes in complete remission was recently evaluated in the H9 F trial [12].
INRT requires greater accuracy in identifying and delineating the involved lymph nodes. Therefore, it is recommended to utilize all modern imaging technologies, particularly pre-chemotherapy PET scans, to achieve this goal. Fusion techniques are recommended to delineate the initial tumor volume on post-chemotherapy CT scans, and all radiological imaging should ideally be performed with the patient in the treatment position. The use of INRT, compared to IFRT, is expected to result in better sparing of normal tissues such as salivary glands, heart, coronary arteries, and breasts in female patients, provided that the initial tumor mass is not too large and the involved lymph nodes are not too numerous [58–60].
The role of radiotherapy in the treatment of thymic neoplasms remains controversial due to conflicting conclusions from multiple small series and the absence of large prospective, randomized trials. Conducting such a trial is challenging due to the low incidence and indolent course of thymic neoplasms, as well as the rapid advancements in RT technology. Currently, the role of adjuvant radiotherapy in the treatment of thymic neoplasms largely depends on the stage of the disease and the extent of surgical resection. Patients with stage I or completely resected stage II disease do not seem to derive significant benefits in terms of survival, local control, or recurrence from the addition of radiotherapy after surgical resection [61–63].
Thymic neoplasms with favorable histology, such as WHO class A, AB, and B1, have also been shown not to benefit from adjuvant radiotherapy. However, patients with stage III and IV disease have high recurrence rates, and certain studies have established the benefits of radiotherapy as part of multimodality therapy, despite some conflicting reports. Additionally, patients with an incomplete (R1 or R2) surgical resection may also benefit from adjuvant radiotherapy [62, 63].
A study by Hsu et al. analyzed 26 patients with thymic carcinoma who received adjuvant radiotherapy and demonstrated improved survival and local control, with a 5-year survival rate of 77%, a 5-year local control rate of 91%, and a 5-year distant metastasis-free rate of 57% [52]. In a small series of 27 patients with invasive thymoma and 6 patients with thymic carcinoma, the radiation dose was found to be important for local control probability [57]. Hsu et al. also concluded that while Masaoka staging was a statistically significant prognostic factor, the radiation dose of < 60 Gy, 60 Gy, or > 60 Gy was not a significant predictor of the overall survival rate [52]. Ogawa et al. reported a 100% control rate in 40 patients with thymic carcinoma who underwent complete resection and received adjuvant radiotherapy with prescription doses exceeding 50 Gy [56].
In a larger multi-institutional series involving 186 thymic carcinoma patients, Kondo et al. found no survival benefit of adjuvant radiotherapy after subtotal resection. The 5-year survival probability for patients with R0 disease who received adjuvant radiotherapy was 73.6%, whereas patients receiving adjuvant chemotherapy, adjuvant chemoradiotherapy, and no adjuvant therapy had 5-year survival probabilities of 81.5%, 46.6%, and 72.2%, respectively [59]. Although various chemotherapeutic regimens, radiotherapy doses, and selection biases may explain these results, they are derived from the largest retrospective series and should be considered by those advocating adjuvant radiotherapy or chemoradiotherapy for all cases of thymic carcinoma.
Due to the rarity of thymic carcinomas and the small number of patients treated, evaluating the effectiveness of chemotherapy is challenging. Most series have treated patients with cisplatin-based regimens similar to those used in the treatment of thymoma [60–62]. Magois et al. presented a series of 9 patients with stage III and IV thymic carcinoma that demonstrated the efficacy of neoadjuvant chemotherapy followed by surgical resection and adjuvant radiotherapy or chemoradiotherapy [63]. Other oncologists suggest that surgery should remain the initial treatment option for clearly resectable, well-defined disease, and radiotherapy should be administered postoperatively if indicated by surgical staging or pathological examination of the surgical specimen [59, 64]. Some studies suggest that neoadjuvant chemotherapy or radiotherapy should be administered only in cases of unresectable thymic carcinoma [58, 65].
Conclusions
INRT is anticipated to exhibit comparable efficacy to IFRT concerning local control, while offering the advantage of significantly reducing late toxicities by limiting the irradiation of normal tissues. The implementation of INRT guidelines will necessitate comprehensive training and robust quality assurance programs to ensure proper execution. For early stage thymomas, surgery remains the established standard of care. The role of RT in stage II patients remains controversial; however, in selected individuals with high-risk features, it has demonstrated a reduction in local recurrence rates. In cases of unresectable thymomas, RT can be safely administered using IMRT with image guidance (IGRT). The utilization of adaptive RT techniques holds promise for further minimizing the dosage delivered to critical normal structures and associated toxicities. Additional research is imperative to establish the dose-response relationship and evaluate the RT-related toxicity of thymomas. Vigilant patient follow-up is crucial for early identification of recurrences and the assessment of late toxicities. A large-scale, multi-center randomized prospective trial is necessary to develop evidence-based guidelines for the management of thymic neoplasms. Furthermore, there remains a lack of consensus regarding the optimal dose and fractionation for thymic carcinoma. Existing studies have employed total doses ranging from 40 Gy to 70 Gy, administered in daily fractions of 1.8 to 2.0 Gy. Looking ahead, future prospects involve advancing our understanding of the therapeutic outcomes and refining treatment approaches for mediastinal neoplasms through ongoing research endeavors and the accumulation of robust clinical evidence.